

| 81g |
| 1g/ml |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢¶¼ŗ¬ÓŠĒāŌŖĖŲ |
| B”¢ÄܽāĄė³öH+ |
| C”¢ÄÜÓė¼ī·¢ÉśÖŠŗĶ·“Ó¦ |
| D”¢¶¼ÄܽāĄė³öĖįøłĄė×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
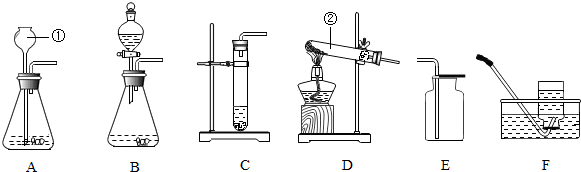
 ³õÖŠ»Æѧ³£¼ūĪļÖŹA-GÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Ģõ¼ž¼°ĪļÖŹŅŃĀŌČ„£©£¬ŅŃÖŖAĪŖµ„ÖŹ£»CĪŖĘųĢ壬¢ŚĪŖŹµŃéŹŅÖĘČ”ĘųĢåCµÄ·½·Ø£»EŹĒÓĆĮæ×ī“óµÄ½šŹō£¬Ęäŗ¬ĮæŌŚµŲæĒÖŠ¾ÓµŚĖÄĪ»£»FŹĒ×īĒįµÄĘųĢ壮Ķ¼ÖŠ¢Ś·“Ó¦ĪļÖ®Ņ»ŹĒ“óĄķŹÆ
³õÖŠ»Æѧ³£¼ūĪļÖŹA-GÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Ģõ¼ž¼°ĪļÖŹŅŃĀŌČ„£©£¬ŅŃÖŖAĪŖµ„ÖŹ£»CĪŖĘųĢ壬¢ŚĪŖŹµŃéŹŅÖĘČ”ĘųĢåCµÄ·½·Ø£»EŹĒÓĆĮæ×ī“óµÄ½šŹō£¬Ęäŗ¬ĮæŌŚµŲæĒÖŠ¾ÓµŚĖÄĪ»£»FŹĒ×īĒįµÄĘųĢ壮Ķ¼ÖŠ¢Ś·“Ó¦ĪļÖ®Ņ»ŹĒ“óĄķŹÆ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
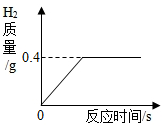 ½«Ņ»¶ØÖŹĮæµÄ½šŹōŠæĶ¶Čėµ½100gĻ”ĮņĖįÖŠĒ”ŗĆĶźČ«·“Ó¦£¬·Å³öĘųĢåµÄÖŹĮæÓė·“Ó¦Ź±¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ŹŌĒó£ŗ
½«Ņ»¶ØÖŹĮæµÄ½šŹōŠæĶ¶Čėµ½100gĻ”ĮņĖįÖŠĒ”ŗĆĶźČ«·“Ó¦£¬·Å³öĘųĢåµÄÖŹĮæÓė·“Ó¦Ź±¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ŹŌĒó£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 øł¾ŻČēĶ¼ÖŠA”¢B”¢CČżÖÖĪļÖŹµÄČܽā¶ČĒśĻߣ¬»Ų“šĻĀĮŠĪŹĢā£®
øł¾ŻČēĶ¼ÖŠA”¢B”¢CČżÖÖĪļÖŹµÄČܽā¶ČĒśĻߣ¬»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com