
��ͼ��ʾ
(A)�ǵ��ˮ�ļ���װ�ã�(B)Ϊ���ˮ�����������(V)��ʱ��(t)�Ĺ�ϵͼ���Իش��������⣮������������A���ҹ���������B(1)
����ͼ�б����Դ��������a��b��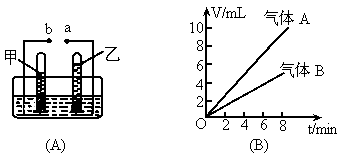
(2)
����B�����õ�ȼ�Ļ����飬��ʲô����˵������B��ʲô���ʣ�(3)
����ʲô�����ռ��������������������������ǵ�ʲô���ʣ�(4)0
��3min����3��6min���ס����������ų�ˮ������ȸ�Ϊ���٣�
|
(1)a Ϊ��Դ������bΪ��Դ������(2)����Ϊ���ȼ�յø�����˵������B������ȼ�ԣ�(3)������ˮ�������������Dz�������ˮ�����ʣ�(4)�ֱ�Ϊ2��1��2��1�����������ĵ缫Ϊ��������������ķ�����ȼ�յ�ľ�������ô����ǵ�ľ��������Ϊ�������������嶼��Ҫ���飬Ӧ��ͬһ������������ͬ�������Թ������岻����ˮ������������������������ˮ���ܽ�Ķ����٣����Կ�������ˮ���ռ��� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
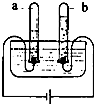 ��ͼ��ʾ���ǵ��ˮʵ��װ�ã�ͨ��һ��ʱ����������Թ��зֱ��ռ�������a������b����ش�
��ͼ��ʾ���ǵ��ˮʵ��װ�ã�ͨ��һ��ʱ����������Թ��зֱ��ռ�������a������b����ش�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
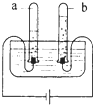 ��ͼ��ʾ���ǵ��ˮʵ��װ�ã�ͨ��һ��ʱ����������Թ��зֱ��ռ�������a������b����ش�
��ͼ��ʾ���ǵ��ˮʵ��װ�ã�ͨ��һ��ʱ����������Թ��зֱ��ռ�������a������b����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��ͼ��ʾ
(A)�ǵ��ˮ�ļ���װ�ã�(B)Ϊ���ˮ�����������(V)��ʱ��(t)�Ĺ�ϵͼ���Իش��������⣮������������A���ҹ���������B(1)
����ͼ�б����Դ��������a��b��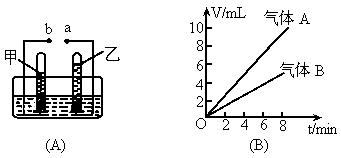
(2)
����B�����õ�ȼ�Ļ����飬��ʲô����˵������B��ʲô���ʣ�(3)
����ʲô�����ռ��������������������������ǵ�ʲô���ʣ�(4)0
��3min����3��6min���ס����������ų�ˮ������ȸ�Ϊ���٣��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com