
| ̼������Ƭ ����ѧ�ɷ֡���Ʒ��Ҫ�ɷ�Ϊ̼�����ƣ���ѧʽΪNaHCO3��������Ϊ84 ����Ч���Ρ����ڻ���θ����������θʹ��θ���ȸУ����ģ������ᣮ ����Ʒ���0.5g/Ƭ ���÷��������ڷ���һ��1��2Ƭ��ÿ��3�Σ� �����ط������ܷ⣬�ڸ��ﴦ���森 |
 =
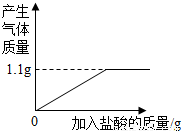
=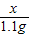 ��� x=2.1��
��� x=2.1�� ×100%=70%��
×100%=70%�� =
=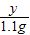 ��� y=0.9125��
��� y=0.9125��

 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ̼������Ƭ ����ѧ�ɷ֡���Ʒ��Ҫ�ɷ�Ϊ̼�����ƣ���ѧʽΪNaHCO3��������Ϊ84 ����Ч���Ρ����ڻ���θ����������θʹ��θ���ȸУ����ģ������ᣮ ����Ʒ���0.5g/Ƭ ���÷��������ڷ���һ��1��2Ƭ��ÿ��3�Σ� �����ط������ܷ⣬�ڸ��ﴦ���森С��ȡ6Ƭ��θҩ���ձ��У������м���������ϡ���ᣨ��θҩ�������ɷֲ�����ˮ��Ҳ�����ᷴӦ���������������������ͼ��ʾ�� 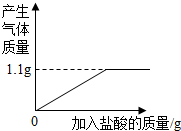 ����㣺 ��1����θҩ��̼�����Ƶ����������Ƕ��٣� ��2�������������ø�ҩƬһ�죬��౻��ȥ��θҺ��HCl�������Ƕ��٣� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ������ С��ͬѧ��ⶨһ��θҩ��̼�����Ƶĺ�������ҩƷ��˵���鲿��ժ¼��ͼ1��ʾ��С��ȡ6Ƭ��θҩ���ձ��У������м���������ϡ���ᣨ��θҩ�������ɷֲ�����ˮ��Ҳ�����ᷴӦ���������������������ͼ2��ʾ�� |
| ̼������Ƭ ����ѧ�ɷ֡���Ʒ��Ҫ�ɷ�Ϊ̼�����ƣ���ѧʽΪNaHCO3��������Ϊ84 ����Ч���Ρ����ڻ���θ����������θʹ��θ���ȸУ����ģ������ᣮ ����Ʒ���0.5g/Ƭ ���÷��������ڷ���һ��1��2Ƭ��ÿ��3�Σ� �����ط������ܷ⣬�ڸ��ﴦ���森 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com