
 ČĖĶعż·ĪÓėĶā½ē½ųŠŠĘųĢå½»»»£¬ĻūŗÄæÕĘųÖŠµÄ²æ·ÖŃõĘų£¬Åųö¶žŃõ»ÆĢ¼”¢Ė®ÕōĘųµČĘųĢ壮µ«ČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒĪüČėæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£æĪŖĮĖÖ¤ŹµÕāøöĪŹĢā£¬ÓŠČĖ²ÉÓĆŅŌĻĀ×°ÖĆ½ųŠŠŹµŃé£ØČēĶ¼£©£®
ČĖĶعż·ĪÓėĶā½ē½ųŠŠĘųĢå½»»»£¬ĻūŗÄæÕĘųÖŠµÄ²æ·ÖŃõĘų£¬Åųö¶žŃõ»ÆĢ¼”¢Ė®ÕōĘųµČĘųĢ壮µ«ČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒĪüČėæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£æĪŖĮĖÖ¤ŹµÕāøöĪŹĢā£¬ÓŠČĖ²ÉÓĆŅŌĻĀ×°ÖĆ½ųŠŠŹµŃé£ØČēĶ¼£©£®·ÖĪö £Ø1£©øł¾ŻŹµŃéÄæµÄ£ŗÖ¤ŹµČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒĪüČėæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£¬·ÖĪö×°ÖĆIŗĶIIµÄ×÷ÓĆ£¬Č»ŗóÅŠ¶ĻČĖĪüĘųŹ±»īČūA”¢BµÄæŖŗĻĒéæö£®
£Ø2£©øł¾ŻŹµŃéÄæµÄ£ŗÖ¤ŹµČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒĪüČėæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£¬·ÖĪö×°ÖĆIŗĶIIµÄ×÷ÓĆ£¬Č»ŗóÅŠ¶ĻČĖŗōĘųŹ±»īČūA”¢BµÄæŖŗĻĒéæö£¬ŅŌ¼°¹Ū²ģµ½µÄĻÖĻó£®
£Ø3£©ČĖŗō³öĘųĢåÖŠµÄ¶žŃõ»ÆĢ¼¶ą£¬ÄÜŹ¹IIÖŠµÄ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£»¶ųæÕĘųÖŠµÄ¶žŃõ»ÆĢ¼²»ÄÜŹ¹IÖŠµÄŹÆ»ŅĖ®±ä»ė×Ē£¬“Ó¶ųĖµĆ÷ČĖŗō³öµÄĘųĢåÖŠŗ¬ÓŠµÄ¶žŃõ»ÆĢ¼²»ŹĒĄ“×ŌæÕĘų£¬¶ųŹĒČĖĢåµÄ“śŠ»²śĪļ£®
½ā“š ½ā£ŗ£Ø1£©±¾ŹµŃéµÄÄæµÄŹĒÖ¤Ć÷ČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£®ĖłŅŌĘæIÖŠĖł×°ŹŌ¼ĮµÄ×÷ÓĆŹĒÖ¤Ć÷æÕĘųÖŠµÄ¶žŃõ»ÆĢ¼ŗ¬ĮæŗÜÉŁ£¬²»ÄÜŹ¹³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£»×°ÖĆIIµÄ×÷ÓĆŹĒÖ¤Ć÷ČĖĢåŗō³öĘųĢåÖŠŹĒ·ńÓŠ¶žŃõ»ÆĢ¼£¬ÓėI¶Ō±Č“Ó¶ųµĆ³ö½įĀŪ£®ĖłŅŌĪüĘųŹ±£¬Ó¦“ņæŖA£¬¹Ų±ÕB£¬ĪŅĆĒ擲»µ½ČĪŗĪ±ä»Æ£®
¹Ź“š°øĪŖ£ŗ“ņæŖ£»¹Ų±Õ£»ĪŽ±ä»Æ£®
£Ø2£©±¾ŹµŃéµÄÄæµÄŹĒÖ¤Ć÷ČĖĢåÅųöµÄ¶žŃõ»ÆĢ¼¾æ¾¹ŹĒæÕĘųÖŠŌÓŠµÄ£¬»¹ŹĒČĖĢå“śŠ»µÄ×īÖÕ²śĪļ£®ĖłŅŌĘæIÖŠĖł×°ŹŌ¼ĮµÄ×÷ÓĆŹĒÖ¤Ć÷æÕĘųÖŠµÄ¶žŃõ»ÆĢ¼ŗ¬ĮæŗÜÉŁ£¬²»ÄÜŹ¹³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£»×°ÖĆIIµÄ×÷ÓĆŹĒÖ¤Ć÷ČĖĢåŗō³öĘųĢåÖŠŹĒ·ńÓŠ¶žŃõ»ÆĢ¼£¬ÓėI¶Ō±Č“Ó¶ųµĆ³ö½įĀŪ£®ĖłŅŌŗōĘųŹ±“ņæŖB£¬¹Ų±ÕA£®ĪŅĆĒæÉŅŌ¹Ū²ģµ½×¶ŠĪĘæ£ØII£©ÖŠÓŠĘųÅŻ²śÉś£¬³ĪĒåŹÆ»ŅĖ®æŖŹ¼±ä»ė×Ē£®
¹Ź“š°øĪŖ£ŗ¹Ų±Õ£»“ņæŖ£»×¶ŠĪĘæ£ØII£©ÖŠÓŠĘųÅŻ²śÉś£¬³ĪĒåŹÆ»ŅĖ®æŖŹ¼±ä»ė×Ē£®
£Ø3£©½«ÉĻŹö²Ł×÷·“ø“½ųŠŠ£¬×¶ŠĪĘæ£ØI£©ÖŠĪŽĆ÷ĻŌ±ä»Æ£¬×¶ŠĪĘæ£ØII£©ÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬Ö¤Ć÷ČĖŗō³öµÄĘųĢåÖŠŗ¬ÓŠµÄ¶žŃõ»ÆĢ¼²»ŹĒĄ“×ŌæÕĘų£¬¶ųŹĒČĖĢåµÄ“śŠ»²śĪļ£®¶žŃõ»ÆĢ¼ÓėŹÆ»ŅĖ®·“Ó¦µÄ»Æѧ·“Ó¦Ź½ŹĒ£ŗCO2+Ca£ØOH£©2ØTCaCO3”ż+H2O£®
¹Ź“š°øĪŖ£ŗ׶ŠĪĘæ£ØI£©ÖŠĪŽĆ÷ĻŌ±ä»Æ£¬×¶ŠĪĘæ£ØII£©ÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£»CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O£®
“š°ø£ŗ
£Ø1£©“ņæŖ£»¹Ų±Õ£»ĪŽ±ä»Æ£®
£Ø2£©¹Ų±Õ£»“ņæŖ£»×¶ŠĪĘæ£ØII£©ÖŠÓŠĘųÅŻ²śÉś£¬³ĪĒåŹÆ»ŅĖ®æŖŹ¼±ä»ė×Ē£®
£Ø3£©×¶ŠĪĘæ£ØI£©ÖŠĪŽĆ÷ĻŌ±ä»Æ£¬×¶ŠĪĘæ£ØII£©ÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£»
µćĘĄ “ĖĢāŹĒĄūÓĆŹµŃé¶ŌŗōĪüŹĒ·ń²śÉś¶žŃõ»ÆĢ¼½ųŠŠŃéÖ¤£¬½āĢāµÄ¹Ų¼üŹĒĮĖ½ā×°ÖĆIŗĶIIµÄ×÷ÓĆ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ȔɣĮæ¹ĢĢåŹ£ÓąĪļÓŚŹŌ¹ÜÖŠ£¬ĻņĘäÖŠ¼ÓČė×ćĮæµÄĻ”ŃĪĖį£® | £Ø1£©¹ĢĢå²æ·ÖČܽā£¬ČÜŅŗĪŖĪŽÉ«£® | ¼ŁÉč¢Ł³ÉĮ¢£®[Ą“ |
| £Ø2£©¹ĢĢå²æ·ÖČܽā£¬ÓŠĘųĢåÉś³É£¬ČÜŅŗĪŖĪŽÉ«£® | ¼ŁÉč¢Ś³ÉĮ¢£® | |
| £Ø3£©¹ĢĢå²æ·ÖČܽā£¬ČÜŅŗĪŖĄ¶É«£® | ¼ŁÉč¢Ū³ÉĮ¢£® |
| ŹµŃé×鱚 | I | II | III | IV | V |
| Ņ© Ę· | Al | Fe | Ag | Al | Cu |
| CuO | CuO | CuO | Fe2O3 | Fe2O3 | |
| ĻąĶ¬Ģõ¼žĻĀŹĒ·ń·“Ó¦ | ŹĒ | ŹĒ | ·ń | ŹĒ | ·ń |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 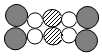 ŅŌ²»Ķ¬ĒņĖµĆ÷·Ö×Ó½į¹¹ | B£® | 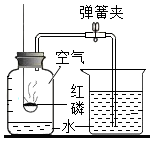 ŅŌĖ®Ī»±ķŹ¾ŃõĘųŗ¬Įæ | ||
| C£® |  ŅŌĖ®ŅųֳɿøßÅŠ¶ĻŅ¶ÕōĢŚæģĀż | D£® |  ŅŌĢśŠ¼ŹżÄæĖµĆ÷“ÅŠŌĒæČõ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2$”ś_{µćČ¼}^{Cl_{2}}$HCl$\stackrel{Na_{2}SO_{4}}{”ś}$NaClČÜŅŗ | |
| B£® | Fe$\stackrel{ŃĪĖį}{”ś}$FeCl3ČÜŅŗ$\stackrel{NaOHČÜŅŗ}{”ś}$Fe£ØOH£©3³Įµķ | |
| C£® | Cu$”ś_{”÷}^{O_{2}}$CuO$\stackrel{Ļ”H_{2}SO_{4}}{”ś}$CuSO4ČÜŅŗ | |
| D£® | CaO$\stackrel{H_{2}O}{”ś}$Ca£ØOH£©2ČÜŅŗ$\stackrel{NaClČÜŅŗ}{”ś}$NaOHČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ČēĶ¼ĖłŹ¾£¬µÆ»É³ĘĻĀŠü¹ŅŅ»ÖŲĪļ£Ø²»ÓėĒāŃõ»Æ±µČÜŅŗŗĶĻ”ĮņĖį·“Ó¦£©£¬ČōĻņĒāŃõ»Æ±µČÜŅŗÖŠÖšµĪµĪ¼ÓĻ”ĮņĖįÖĮĒ”ŗĆĶźČ«·“Ó¦£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖBa£ØOH£©2+CuSO4ØTCu£ØOH£©2”ż+BaSO4”ż£¬·“Ó¦½įŹųŗóµÆ»É³ĘµÄŹ¾Źż½«±ä“󣮣ØĢī±ä“󣬱䊔£¬»ņ²»±ä£©
ČēĶ¼ĖłŹ¾£¬µÆ»É³ĘĻĀŠü¹ŅŅ»ÖŲĪļ£Ø²»ÓėĒāŃõ»Æ±µČÜŅŗŗĶĻ”ĮņĖį·“Ó¦£©£¬ČōĻņĒāŃõ»Æ±µČÜŅŗÖŠÖšµĪµĪ¼ÓĻ”ĮņĖįÖĮĒ”ŗĆĶźČ«·“Ó¦£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖBa£ØOH£©2+CuSO4ØTCu£ØOH£©2”ż+BaSO4”ż£¬·“Ó¦½įŹųŗóµÆ»É³ĘµÄŹ¾Źż½«±ä“󣮣ØĢī±ä“󣬱䊔£¬»ņ²»±ä£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
 ŌŚŃ§Š£µÄŌŖµ©ĮŖ»¶»įÉĻ£¬Ä³Ķ¬Ń§±ķŃŻĮĖ”°Ė®ÄÜÉś»š”±µÄħŹõ£®ĖūĻņ°üÓŠ¹żŃõ»ÆÄĘ£ØNa202£©·ŪÄ©µÄĶŃ֬ƎÉĻµĪĖ®£¬ĶŃÖ¬ĆŽČ¼ÉÕĘšĄ“£®Š”²ĢŗÜøŠŠĖȤ£¬ÓŚŹĒ£¬ĖūŗĶĶ¬Ń§ĆĒ½ųŠŠĢ½¾æ£®
ŌŚŃ§Š£µÄŌŖµ©ĮŖ»¶»įÉĻ£¬Ä³Ķ¬Ń§±ķŃŻĮĖ”°Ė®ÄÜÉś»š”±µÄħŹõ£®ĖūĻņ°üÓŠ¹żŃõ»ÆÄĘ£ØNa202£©·ŪÄ©µÄĶŃ֬ƎÉĻµĪĖ®£¬ĶŃÖ¬ĆŽČ¼ÉÕĘšĄ“£®Š”²ĢŗÜøŠŠĖȤ£¬ÓŚŹĒ£¬ĖūŗĶĶ¬Ń§ĆĒ½ųŠŠĢ½¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Cr2£ØSO4£©3 | B£® | Cr2O3 | C£® | K2Cr2O7 | D£® | Cr£ØOH£©3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ČĖĄąÉś»īĄė²»æŖ½šŹō£®
ČĖĄąÉś»īĄė²»æŖ½šŹō£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com