
| ¼ÓČČŹ±¼ä | t1 | t2 | t3 | t4 |
| Ź£Óą¹ĢĢåµÄÖŹĮæ | 2.12 | 2.08 | 2.04 | 2.04 |
·ÖĪö ĀČĖį¼ŲŌŚ¶žŃõ»ÆĆĢµÄ“ß»Æ×÷ÓĆĻĀ£¬ŹÜČČ·Ö½āÉś³ÉĀČ»Æ¼ŲŗĶŃõĘų£»
·“Ó¦Ē°ŗóµÄÖŹĮæ²ī¼“ĪŖ·“Ӧɜ³ÉŃõĘųµÄÖŹĮ棻
øł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½ŗĶĢį¹©µÄŹż¾ŻæÉŅŌ½ųŠŠĻą¹Ų·½ĆęµÄ¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2KClO3$\frac{\underline{MnO_2}}{”÷}$2KCl+3O2”ü£»
£Ø2£©¼ÓČȵ½t3Ź±¼äŗ󣬹ĢĢåÖŹĮæ²»ŌŁ¼õŠ”£¬ĖµĆ÷ĀČĖį¼ŲŅŃ¾ĶźČ«·“Ó¦£»
£Ø3£©ĶźČ«·“Ӧɜ³ÉŃõĘųµÄÖŹĮæĪŖ£ŗ2.5g+0.5g-2.04g=0.96g£¬
0.96gŃõĘųµÄĪļÖŹµÄĮæĪŖ£ŗ0.96g”Ā32g/mol=0.03mol£»
£Ø4£©ÉčĀČĖį¼ŲµÄÖŹĮæĪŖx£¬
2KClO3$\frac{\underline{MnO_2}}{”÷}$2KCl+3O2”ü£¬
245 96
x 0.96g
$\frac{245}{x}$=$\frac{96}{0.96g}$£¬
x=2.45g£¬
øĆѳʷ֊ĀČĖį¼ŲµÄ“æ¶ČĪŖ£ŗ$\frac{2.45g}{2.5g}$”Į100%=98%£¬
¹Ź“š°øĪŖ£ŗ2KClO3$\frac{\underline{MnO_2}}{”÷}$2KCl+3O2”ü£»ŹĒ£»0.96g£»0.03mol£»2.45g£»98%£®
µćĘĄ ²īĮæ·ØŌŚ¼ĘĖćÖŠµÄÓ¦ÓĆŗܹć·ŗ£¬½ā“šµÄ¹Ų¼üŹĒŅŖ·ÖĪö³öĪļÖŹµÄÖŹĮæ²īÓėŅŖĒóµÄĪ“ÖŖŹżÖ®¼äµÄ¹ŲĻµ£¬ŌŁøł¾Ż¾ßĢåµÄŹż¾ŻĒó½ā£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¾Ę¾«»Ó·¢ | B£® | »šŅ©±¬ÕØ | C£® | ŗ£Ė®É¹ŃĪ | D£® | ³±ŹŖµÄŅĀ·ž±äøÉ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
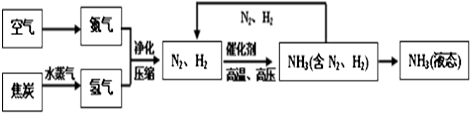
| ĪļÖŹ | H2 | N2 | O2 | NH3 |
| ·Šµć | -252”ę | -195.8”ę | -183”ę | -33.35”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
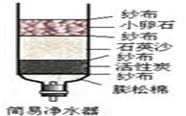 Ė®ŹĒČĖĄąÉś»īÖŠ²»æÉȱɣµÄĪļÖŹ£®
Ė®ŹĒČĖĄąÉś»īÖŠ²»æÉȱɣµÄĪļÖŹ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
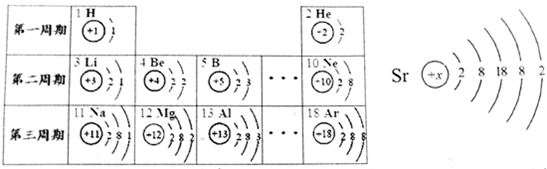
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com