
�����dz��л�ѧ������Ҫ��ʵ��װ�á��밴Ҫ����գ�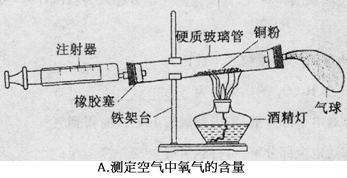
��1��A��Ϊ�˲ⶨ�����������ĺ��������û�ѧ������ȥ�����е�������������Ӧ�Ļ�ѧ����ʽ�� ����װ�����������ã�ʵ������������ĺ���С��21%����ԭ����ܢ� ���� ��
��2��Bʵ��a�п����������� ���û�ѧ����ʽ��ʾ�������ԭ�� ��bװ���е�������˵��������̼���� ���ʣ���һ������ʵ�������е�Ӧ��Ϊ ��
��3��С����Cͼʵ��ʱ������ƿ�ײ�ը�ѣ�����ܵ�ԭ���� ��
С������˿��������ȼ��Ϊʲô������������̽��������þ�Ͳ�ͬ��̼��������þ����ֱ����Ϊ0��4mm������������ȼ�գ����������¼���±��С�
| ���� | þ | ��̼0��05%���� | ��̼0��2%���� | ��̼0��6%���� |
| ȼ��ʱ ������ | ����ȼ�գ����� ҫ�۰⣬���� | ����ȼ�� ���ٻ��� | ����ȼ�� �������� | ��δ� |
��1��2Cu + O2 2CuO
2CuO
��ͭ�۵���̫�٣�����δ������
��δ��ȴ�����¾Ͷ�����������ע������������̫�٣�û��ʹ�ܱ����������������ͭ��Ӧ��������¶Ȳ����ߣ�ͭ����������Ӧ����ֵȵȣ�
��2����ɫʯ����Һ��Ϊ��ɫ H2O+CO2===H2CO3 ������ȼ�ա���֧��ȼ�ա��ܶȱȿ����� ���
��3������ƿ��û�з�����ˮ����һ��ϸɳ
�پ���ȼ�գ��������� ����˿�к��е�̼�����
���������������1��Ӳ�ʲ���������ͭ��ͭ����������������ͭ�Գ�ȥ�����������ͭ�۲����δ��ȴ�����¾Ͷ����������ע������������̫�٣�û��ʹ�ܱ����������������ͭ��Ӧ��������¶Ȳ����ߣ�ͭ����������Ӧ����ֵȵȾ��ᵼ�²ⶨ�������ĺ���ƫ�٣�
��2��������̼��ˮ��Ӧ����̼�ᣬ̼������ԣ���ʹ��ɫʯ����Һ��Ϊ��ɫ��b������Ϩ�����˵��������̼�Ȳ���ȼ��Ҳ����֧��ȼ�գ�
��3����ȼ��ʱ���������¶Ⱥܸߣ�������ƿ��û�з�����ˮ����һ��ϸɳ����ƿ��ը�ѣ����ݱ��������֪����̼��Խ�ߣ�ȼ��Խ���ң���ô����δ���ʵ�������Ǿ���ȼ�գ��������䡣
���㣺�����ɷ�ʵ�顢������̼����ʵ�顢��������ʵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ�����ú�ˮ��ȡ���εĹ��̣�
��1�����ݺ�ˮɹ�ε�ԭ��������˵������ȷ�������� ��������ĸ����
| A����ˮ������ˮ�أ���ˮ�ijɷֻ������䣻 |
| B�����������У���ˮ���Ȼ��Ƶ����������ӣ� |
| C�����������У���ˮ��ˮ�����������ӣ� |
| D������������ĸҺ���Ȼ��Ƶı�����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�ҹ���һ��ƶˮ�Ĺ��ҡ������������ȣ��ҹ�ˮ��Դ�������ڰ���������˹��������ӡ�������ǣ�λ�������6λ�������˾�ˮ��Դ���㣬���Ϊ����ˮƽ��1/4���ڵ�110λ֮��ȱˮ�����ȫ����Χ���ձ���ڣ������в��ϼӾ�����ơ�����ˮ����Ⱦ�൱���أ�ˮ��Ⱦ��Ҫ������Щ���棿˵�������ճ������Լ��ˮ��һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�ӷ��ӵĽǶȷ����������������⣮
��1����ů�������������磮�� ��
��2��Һ��ʯ������ѹ���ڸ�ƿ�У��� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ʵ������������������Ϊ10%����������Һ����ˮ�ĵ��ʵ�飮
ʵ��һ������100g������������Ϊ10%����������Һ��
��1����������������������� ��g��
��2������ʱ���������ƹ���Ӧ������ƽ�� ��������ҡ����̵��ձ��ڣ���ȡ�����ˮ��ˮ���ܶ�Ϊ1g/cm3����Ӧѡ�����Ͳ�Ĺ������ ��mL��ѡ�10������100����250������
ʵ�����ˮ�ĵ��ʵ��
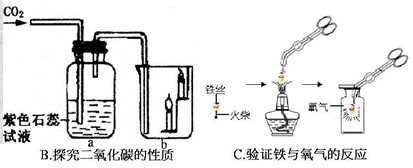
����ͼװ�ý���ˮ�ĵ��ʵ�飮��֪����������ˮ�ĵ��ʵ���У�ֻ����ǿˮ�ĵ��������ã�
��3����ʼ��Ӧǰa��b�����ڶ�������Һ���رջ�������ͨ��Դ��һ��ʱ������ܲ�����������ͼ��ʾ����a���·�����Ӧ�ӵ�Դ�� ����������������������� ����֤b���е����壬д�����ˮ�Ļ�ѧ����ʽ���� ��
��4��˵��ˮ��һ�ֻ������ʵ����ʵ���� ��
��5��������Һ���������������� ��10%�����������=����������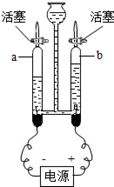
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��8�֣���������ʵ��װ��ͼ���ش��й����⣺

��1��д��ͼ�б�����������ƣ��� ���� ��
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ�� ��ѡ�õ��ռ�װ���� ��ѡ����ĸ����
��3��ʵ���ҿ��������ƹ�����Ũ��ˮ�ڳ����»����ȡ����������Ϊ�˵õ�ƽ�ȵİ�������ѡ�õķ���װ���� ��ѡ����ĸ����
��4������ͼ����ʾװ��������������ȼ�յ�ʵ�飬ȼ�ս�����ȡ��ȼ�ճף������ò���Ƭ��סƿ�ڲ�����������ƿ��ת�����ֲ���Ƭ������ס����������������ԭ����
��Ϊ�������ʵ�飬��10mL��Ͳ��ȡ6.0mL����������Һ������ʱ��Ͳ�ڵ�Һ�尼Һ����ʹ�Ӧ����ͼ���� ���̶��߱���ˮƽ��ѡ�a����b������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
������ʵ������ȡ���峣�õĻ�ѧ��������ش��������⣮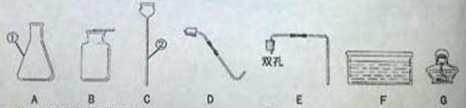
��1��д��ָ�����������ƣ�����_________��������_________����
��2��ʵ������ȡ������̼ʱ��Ӧѡ�õ���������_________��������ĸ�����йط�Ӧ�Ļ�ѧ����ʽ����_________����ijͬѧ��ȼ�ŵ�Сľ�����ڼ���ƿ�ڣ�ʼ��δ����Сľ��Ϩ�𣬳������������ԭ���������_________����д��һ������
��3��ʵ�����������ṩ��������ȡ��������Ӧ�Ļ�ѧ����ʽ����_________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
�ס��ҡ����dz��л�ѧ�г��������ֲ�ͬ���͵����ʣ���������ˮ������֮���ת����ϵ��ͼ��ʾ����������ʾ��һ������ת������һ�����ʣ�ͼ�в��ַ�Ӧ������������ȥ����
��1����������������� ����
��2��д����ת��Ϊ���Ļ�ѧ����ʽ�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ijͬѧ���������˽������̼������ˮ����ˮ������Ӧ�����ᡣ��ͬѧ���������ʵ�飬��֤����һ���ۡ�
ʵ��l��ȡһС�龭��ɫʯ����Һ���ݹ��ĸ����ֽ(���¼�ơ�ֽ��)����ֽ�ϵ��ϼ���ϡ���ᣬֽ���ɫ��ʵ��2��ȡһС��ֽ����ֽ�ϵ��ϼ��δ�����ˮ��ֽ����ɫ��
ʵ��3��
ʵ��4����������̼ͨ��ʢ��ˮ���Թ���һ������ý�ͷ���ȡ���е���Һ�ε�ֽ�ϣ�ֽ���
�ش��������⣺
��1��ʵ��2��Ŀ���ǣ� ��
��2������ʵ��3�� ��
��3��д��������̼��ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com