
| NaCl | KCl | NH4Cl | KNO3 | |
| 10�� | 35.8g | 31.0g | 33.3g | 20.9g |
| 60�� | 37.3g | 45.5g | 55.2g | 110.0g |
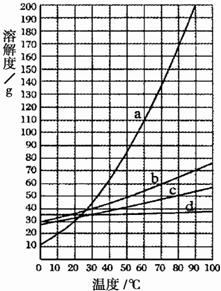
| A��b���ܽ��С��a���ܽ�� |
| B��ͼ��a���߱�ʾ����ص��ܽ������ |
| C��Ҫ��a��d�Ļ�����еõ�a��ͨ�����������ܼ�ʹ��ᾧ�ķ��� |
| D���Ȼ��صIJ�������Һ��60�潵����l0��ʱ��ɱ�����Һ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?�ijǣ���ͼ��a��b��c��d���ֹ������ʵ��ܽ�����ߣ��±�����Щ���������ڲ����¶�ʱ���ܽ�ȣ� ��2009?�ijǣ���ͼ��a��b��c��d���ֹ������ʵ��ܽ�����ߣ��±�����Щ���������ڲ����¶�ʱ���ܽ�ȣ�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 20����ͼ��a��b��c��d���ֹ������ʵ��ܽ�����ߣ�ͼ������Щ���������ڲ����¶�ʱ���ܽ�ȣ�����ͼ����Ϣ�ж�����˵����ȷ���ǣ������� 20����ͼ��a��b��c��d���ֹ������ʵ��ܽ�����ߣ�ͼ������Щ���������ڲ����¶�ʱ���ܽ�ȣ�����ͼ����Ϣ�ж�����˵����ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��A��B��C��D���ֹ������ʵ��ܽ������ͼ�����ݴ�ͼ����д���и��գ�
��ͼ��A��B��C��D���ֹ������ʵ��ܽ������ͼ�����ݴ�ͼ����д���и��գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com