
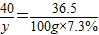

 ×100%=8%
×100%=8%

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø10ÖŲĒģōė½24£©Š”ĒąĶ¬Ń§ĪŖĮĖÖĘ×÷Ņ¶ĀöŹéĒ©£¬“ņĖćÅäÖĘ125g10£„µÄNaOHČÜŅŗ”£
£Øl£©¼ĘĖ抔ĒąŠčŅŖ³ĘČ”NaOHµÄÖŹĮ攣
£Ø2£©Š”Ēą½«Ļ“¾»µÄŹ÷Ņ¶·ÅŌŚÅäÖĘŗƵÄČÜŅŗÖŠÖó·ŠŗóČ”³ö£¬ČÜŅŗµÄÖŹĮæ¼õÉŁĮĖ5g£¬ČÜÖŹÖŹĮæ·ÖŹżŅ²ÓŠĖł¼õŠ””£ĪŖĮĖ²ą¶ØŹ£ĻĀČÜŅŗÖŠNaOHµÄÖŹĮæ·ÖŹż£¬Š”ĒąĻņČÜŅŗÖŠÖš½„¼ÓČė7.3£„µÄĻ”ŃĪĖį£¬µ±ČÜŅŗPH=7Ź±£¬ĻūŗÄŃĪĖį50g”£¼ĘĖć£ŗ
¢ŁÉś³ÉNaClµÄÖŹĮ攣
¢ŚŹ£ĻĀČÜŅŗÖŠNaOHµÄÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com