
����Ŀ��ijУ��ѧ�о���ѧϰ�������ͬѧ��Ϊ�˲ⶨij����������Ʒ��Ca��OH��2���������е�����ΪCaCO3����������3��ʵ�飬ʹһ�������ĸ������ֱ���ͬһ�����ᷴӦ���������ʵ�����ݼ�¼���±���
��1�� | ��2�� | ��3�� | |
��ȡ��Ʒ������ | 17.4g | 17.4g | 17.4g |
��ȡϡ��������� | 80g | 100g | 120g |
����CO2������ | 2.64g | 4.4g | 4.4g |
��1���ڵ���ʵ���У���Ӧ�������а�ɫ����ʣ�࣮
��2����ԭ������Ca��OH��2������������
���𰸡�
��1��1
��2���⣺�������Ʒ�к�̼��Ƶ�����ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
X 4.4g
���ݣ� ![]() �����X=10g
�����X=10g
Ca��OH��2���������� ![]() ��100%��42.5%
��100%��42.5%
���������⣺��1����һ�κ͵ڶ����м�����Ʒ������ͬ���ڶ��μ�����������ˣ����ɶ�����̼���������ˣ�˵����һ�η�Ӧ���̼�����ʣ�࣬���ݵ����μ�������������ˣ������ɶ�����̼����û�䣬˵���ڶ��η�Ӧ�̼꣬���û��ʣ�࣮
��ԭ������Ca ��OH��2����������Լ��42.5%
�ʴ�Ϊ����1��1����2��42.5%��
��1�����ݵ�һ�κ͵ڶ������ݷ�������Ʒ����һ��������������ˣ����ɶ�����̼���������ˣ�˵���˵�һ�η�Ӧ���̼�����ʣ�ࣻ��2�����ݶ�����̼�������������̼��Ƶ�������������Ʒ������ȥ̼��Ƶ���������������Ƶ��������ٳ�����Ʒ���������ɣ�


 ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��������ͼ��ʾ 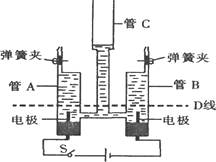 �ļ����ˮװ�ã����м�ͥСʵ��(ע����װ�����������ã��ҷ�Ӧһ��ʱ���ֹͣͨ�磬A��B����Һ�������ͼ��D��).�����Ҫ��ش����⣺
�ļ����ˮװ�ã����м�ͥСʵ��(ע����װ�����������ã��ҷ�Ӧһ��ʱ���ֹͣͨ�磬A��B����Һ�������ͼ��D��).�����Ҫ��ش����⣺
��1���պϿ��غ�۲쵽��A��B���ڵ�������.��C���е������� �� �����������ԭ����.
��2��A��B�������ɵ�����ۼ����ϲ���ԭ����.
��3��������A�������ɵ�����Ӧ���� .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼʵ��װ��ʾ��ͼ���ش��й����⣺
��1��д��ͼ�б��a���������ƣ�
��2��д��A�з�����Ӧ�Ļ�ѧ����ʽ�� �� B��װ����Լ���
��3����D�ռ������壬����ˮ��������
��4��ͨ��E��F����ʵ�飬��Ͽ���ѧϰ������Ϊ�ڡ�����ƿ�н���ȼ�յ�ʵ�顱Ӧ��ע��������ǣ�˵�����㼴�ɣ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������еı仯����ֻ���������仯���ǣ� ��
A.������������ȡ������
B.��һ�������£���ʯī������ʯ
C.������̼���徭���£���ѹ�Ƶøɱ�
D.�ŷŵ������еĶ��������γ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G�dz������ʣ�B��һ�����嵥�ʣ�E�Ǻ�ɫ�Ĺ��嵥�ʣ�D��dz��ɫ��Һ��G����ɫ��Һ�����ǿ��Է�������ת����ϵ�� 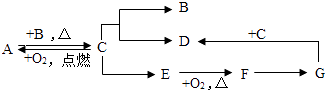
��1���Ƴ��������ʵĻ�ѧʽ��A��B��D��G��
��2��д������ת���Ļ�ѧ����ʽ��E��F����Fת��ΪG�ķ�Ӧ�û���Ӧ����ǡ�������һ���ǡ����ǡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������¶�ÿ�����������С���ǣ� ��
A.Ũ����
B.Ũ����
C.��������
D.�Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڻ�ѧ������û�������İ���������Ҳ����ʻ������������ڲ�ͬ��λ�ñ�ʾ�Ų�ͬ�ĺ��壮���л�ѧ���������֡�2����ʾ��������ȷ���ǣ� ��
A.Mg2+��һ��þ���Ӵ�2����λ�����
B.![]() �������ƵĻ��ϼ�Ϊ+2��
�������ƵĻ��ϼ�Ϊ+2��
C.2H��2����Ԫ��
D.CO2��������̼�����к�����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪M��N�ֱ���ϡ���ᡢ����������Һ�е�һ�֣�ij��Ȥʵ��С��ͬѧ��һ������M�в��ϵμ�N�����ⶨ������Һ��pH����ͼ��ʾ�� 
��1��M�������������ƣ���
��2����Ӧ�����У���ʦȡa��b��c������Ӧ�����Һ������˳�����ͬѧ�Dz��ⶨ��Һ��pHֵ��������������������Һ����̽����
С����С��ֱ�ȡ����һ����Һ����ʵ�飺
С������ȡ��Һ�м�����Һ���۲쵽����ɫ�������ɣ�
���ۣ���a����Һ��
С����������һ����Һ�еμӷ�̪��Һ���۲쵽 ��
���ۣ���b����c����Һ��
Ϊ��һ��ȷ������Һ�ɷ֣�С������Ʋ��������ʵ�飺
ʵ�鲽�� | ���� | ���� |
Ϊc����Һ������֪��Һ�е� |
ʣ��һ��Ϊb����Һ��
ͨ��ʵ�飬ͬѧ��֪������Ӧ������pH�ı仯���ò�ͬ����ȷ����Һ�ɷ�
��3����Ȥʵ��С��ͬѧͨ��ʵ�����飬ij�����ķ���Һ��Ⱦ����ˮ���������������Ϣ����ѡ���������ʴ�����ˮ��
���� | ��Է������� | �г��ο���ֵ��Ԫ/Kg�� |
CaCO3 | 100 | 1.8 |
Ca��OH��2 | 74 | 2.0 |
NaOH | 40 | 11.5 |
���Ҫ�����ٵ�Ǯ����Ӧͬ�����ķ����ᣬ��Ӧѡ���������ʻ�ѧʽ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com