
 £»
£»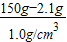 =147.9mL
=147.9mL


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
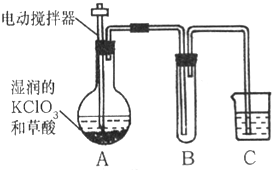
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 £Ø2006?ŃĢĢØ£©ÓĆĀČĘųĻū¶¾ŅūÓĆĖ®Ź±£¬»įÉś³ÉÉŁĮæ¶ŌČĖĢåÓŠŗ¦µÄÓŠ»śĪļ£®Ņņ“Ė£¬ŹĄ½ē»·±£ĮŖĆĖ½ØŅéČ«Ćę½ūÖ¹ÓĆĀČĘų¶ŌŅūÓĆĖ®Ļū¶¾£¬ĶĘ¹ćŹ¹ÓĆ°²Č«”¢øߊ§É±¾śĻū¶¾¼ĮClO2£®ClO2ŹĒŅ»ÖÖ»ĘĀĢÉ«”¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬ĄäČ“ÖĮ11.0”ęŅŌĻĀŹ±±ä³ÉŗģÉ«ŅŗĢ壬Ņ×ČÜÓŚĖ®£¬¼ū¹āŅ×·Ö½ā£®ClO2Ņ×Óė¼ī·“Ó¦£¬Ęäɱ¾ś”¢ĘÆ°×ÄÜĮ¦¾łÓÅÓŚĀČĘų£¬Ļū¶¾Ė®ĢåŹ±²»Éś³ÉÓŠŗ¦ĪļÖŹ£¬Ņ²²»“ęŌŚÓĆĀČĘųĻū¶¾Ź±²ŠĮōµÄĘųĪ¶£®
£Ø2006?ŃĢĢØ£©ÓĆĀČĘųĻū¶¾ŅūÓĆĖ®Ź±£¬»įÉś³ÉÉŁĮæ¶ŌČĖĢåÓŠŗ¦µÄÓŠ»śĪļ£®Ņņ“Ė£¬ŹĄ½ē»·±£ĮŖĆĖ½ØŅéČ«Ćę½ūÖ¹ÓĆĀČĘų¶ŌŅūÓĆĖ®Ļū¶¾£¬ĶĘ¹ćŹ¹ÓĆ°²Č«”¢øߊ§É±¾śĻū¶¾¼ĮClO2£®ClO2ŹĒŅ»ÖÖ»ĘĀĢÉ«”¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬ĄäČ“ÖĮ11.0”ęŅŌĻĀŹ±±ä³ÉŗģÉ«ŅŗĢ壬Ņ×ČÜÓŚĖ®£¬¼ū¹āŅ×·Ö½ā£®ClO2Ņ×Óė¼ī·“Ó¦£¬Ęäɱ¾ś”¢ĘÆ°×ÄÜĮ¦¾łÓÅÓŚĀČĘų£¬Ļū¶¾Ė®ĢåŹ±²»Éś³ÉÓŠŗ¦ĪļÖŹ£¬Ņ²²»“ęŌŚÓĆĀČĘųĻū¶¾Ź±²ŠĮōµÄĘųĪ¶£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com