
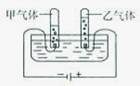
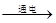 2H2��+O2�������ʵ���������
2H2��+O2�������ʵ���������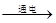 2H2��+O2�����ڱ�״���·��Ӹ����������ʵ��������ȣ�������������ˮ�е��ܽ����ϴ������������������������ȿ��ܴ���2��1
2H2��+O2�����ڱ�״���·��Ӹ����������ʵ��������ȣ�������������ˮ�е��ܽ����ϴ������������������������ȿ��ܴ���2��1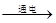 2H2��+O2�������ʵ��������ڣ�
2H2��+O2�������ʵ��������ڣ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ʱ�䣨min�� | 0 | 3 | 6 | 9 |
| ��п����Һ��������g�� | 150.4 | 150.2 | 150.0 | 150.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
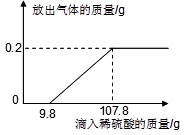
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
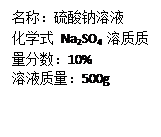
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
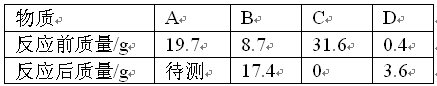
| A��Cһ���ǻ����D�����ǵ��� |
| B����Ӧ�����У�B��D�仯��������Ϊ87��36 |
| C����Ӧ���ܱ�������A������Ϊ19.7 g |
| D����Ӧ��A��C�Ļ�ѧ������֮��Ϊ1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com