ʵ���ҳ���ϡ�����ʯ��ʯ��Ӧ��ȡ������̼��
��1���÷�Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+CO2��+H2O
CaCO3+2HCl=CaCl2+CO2��+H2O
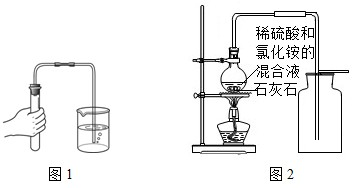
��2��ͼ1��ʾ����ҩƷǰ��һ����������Ŀ����
Ϊ��ֹ���岻�������ռ�
Ϊ��ֹ���岻�������ռ�
��
��3��д���������ɵ������Ƕ�����̼�Ļ�ѧ����ʽ��
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
��
��4��ʵ����ͨ����ѡ��ϡ�����ʯ��ʯ��Ӧ��ȡ������̼�������ɵ���������ƻ��谭��Ӧ�Ľ�һ�����У�ijС�龭�о�����ͼ2��ʾ�����ɹ��Ƶ��˴���CO
2����Ӧ��Ϊϡ�����ʯ��ʯ����
��ʹ��ͼ���ռ�������̼������ԭ��
������̼����������ˮ���ܶȱȿ�����
������̼����������ˮ���ܶȱȿ�����
��
�ڸ�װ����ƿ����Ȧ�����ʯ������������
ʹ��ƿ���Ⱦ��ȣ���ֹը��
ʹ��ƿ���Ⱦ��ȣ���ֹը��
��
���Ʋ⣺��������Һ�м����Ȼ�鱗�����Һ���ȣ�Ŀ�Ķ���
�������������Һ�е��ܽ��
�������������Һ�е��ܽ��
��
����ͬѧ�������������ӷ��ԵIJ��죬��Ϊͼ2���������ϡ�����ʯ��ʯ��Ӧ���Ľ����ŵ���
ԭ�����е�������лӷ��ԣ��������̼��������Ų�������ʵ������
ԭ�����е�������лӷ��ԣ��������̼��������Ų�������ʵ������
��
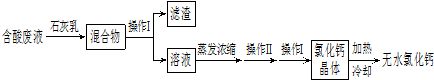
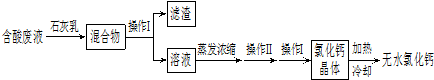
��5���Ȼ����Ƕ���;�ĸ�����ͽ�����������ʵ��������ʯ��ʯ�������Ʊ�������̼�ĺ����Һ������MgCl
2��FeCl
3�ȣ���ͨ������;���Ƶ���ˮ�Ȼ��ƣ�
�ٲ��� I������Ϊ
����
����
�������Ļ�ѧ�ɷ���
Mg��OH��2��Fe��OH��3
Mg��OH��2��Fe��OH��3
��
���û�ѧ����ʽ��ʾʯ��������ã�дһ����
�кͷ�Һ�е���
�кͷ�Һ�е���
��



 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�

 ʵ���ҳ���ϡ�����ʯ��ʯ��Ӧ��ȡ������̼��
ʵ���ҳ���ϡ�����ʯ��ʯ��Ӧ��ȡ������̼��