
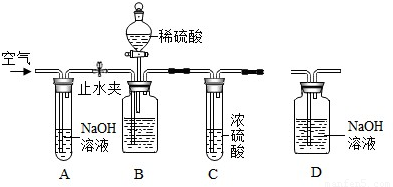
 ��ҵ�����г�����������NaCl���ʣ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã����������ã���Ʒ����Ϊ11.0g��װ��D������Ϊ172.2g������������Ϊ������Ʒװ����ƿ�С���ֹˮ�У�����������������ӡ�����װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ�еμ�ϡ���������ٲ������ݡ���ֹˮ�У��ٻ���������������ӡ�����װ��D������Ϊ176.6g��������ÿ��װ�þ���Ӧ��ȫ����
��ҵ�����г�����������NaCl���ʣ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã����������ã���Ʒ����Ϊ11.0g��װ��D������Ϊ172.2g������������Ϊ������Ʒװ����ƿ�С���ֹˮ�У�����������������ӡ�����װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ�еμ�ϡ���������ٲ������ݡ���ֹˮ�У��ٻ���������������ӡ�����װ��D������Ϊ176.6g��������ÿ��װ�þ���Ӧ��ȫ����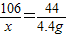
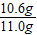 ×100%=96.4%
×100%=96.4% 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
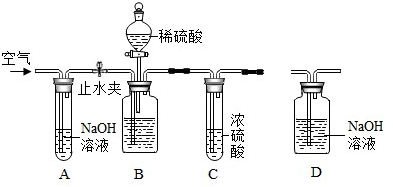 ��2012?�����ʼ죩��ҵ�����г�����������NaCl���ʣ�����ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4=Na2SO4+H2O+CO2������װ�����������ã�������Ʒ����Ϊ11.0g��װ��D��Ӧǰ������Ϊ172.2g����
��2012?�����ʼ죩��ҵ�����г�����������NaCl���ʣ�����ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4=Na2SO4+H2O+CO2������װ�����������ã�������Ʒ����Ϊ11.0g��װ��D��Ӧǰ������Ϊ172.2g�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��̨����ģ����ҵ�����г�����������NaCl���ʣ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã����������ã���Ʒ����Ϊ11.0g��װ��D������Ϊ172.2g������������Ϊ������Ʒװ����ƿ�С���ֹˮ�У�����������������ӡ�����װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ�еμ�ϡ���������ٲ������ݡ���ֹˮ�У��ٻ���������������ӡ�����װ��D������Ϊ176.6g��������ÿ��װ�þ���Ӧ��ȫ����
��2012?��̨����ģ����ҵ�����г�����������NaCl���ʣ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ�ã����������ã���Ʒ����Ϊ11.0g��װ��D������Ϊ172.2g������������Ϊ������Ʒװ����ƿ�С���ֹˮ�У�����������������ӡ�����װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ�еμ�ϡ���������ٲ������ݡ���ֹˮ�У��ٻ���������������ӡ�����װ��D������Ϊ176.6g��������ÿ��װ�þ���Ӧ��ȫ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�츣��ʡ�����о��꼶��ҵ��������鿼�Ի�ѧ�Ծ����������� ���ͣ�������
��ҵ�����г�����������NaCl���ʡ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ �ã���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3 + H2SO4 Na2SO4 + H2O + CO2������װ�����������ã� ������Ʒ����Ϊ11.0 g��װ��D��Ӧǰ������Ϊ172.2 g����
����������
�ٽ���Ʒװ��Bװ�ù��ƿ�С���ֹˮ�У�
����������������ӣ�
������װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ
�еμ�ϡ���������ٲ������ݣ�
�۴�ֹˮ�У��ٴλ���������������ӣ�
�ܳ�����Ӧ��װ��D������Ϊ176.6 g��������ÿ��װ�þ���Ӧ��ȫ����
��1����ʵ�������װ��D���ն�����̼ g�����㴿����Ʒ��Na2CO3������������
�����ȥ��Cװ�ã���ⶨ��� ������ţ���
�� �� ��ƫС �۲���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�����о��꼶��ҵ��������鿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��ҵ�����г�����������NaCl���ʡ���ͼ�Dzⶨ������Ʒ��Na2CO3����������ʵ��װ �ã���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3 + H2SO4 Na2SO4 + H2O + CO2������װ�����������ã� ������Ʒ����Ϊ11.0 g��װ��D��Ӧǰ������Ϊ172.2 g����
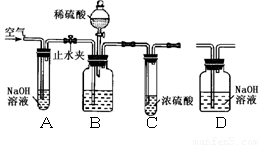
����������
�ٽ���Ʒװ��Bװ�ù��ƿ�С���ֹˮ�У�
����������������ӣ�
������װ��D���ر�ֹˮ�У���ʢ����Ʒ�Ĺ��ƿ
�еμ�ϡ���������ٲ������ݣ�
�۴�ֹˮ�У��ٴλ���������������ӣ�
�ܳ�����Ӧ��װ��D������Ϊ176.6 g��������ÿ��װ�þ���Ӧ��ȫ����
��1����ʵ�������װ��D���ն�����̼ g�����㴿����Ʒ��Na2CO3������������
�����ȥ��Cװ�ã���ⶨ��� ������ţ���
�� �� ��ƫС �۲���
����������1���������֪������̼������=176.6 g-172.2 g=4.4 g�����ݻ�ѧ����ʽ���Na2CO3���������н��
��2��Ũ���������ˮ���������������������̼�ģ��������Ũ���ᣬ�ͻὫ������̼�е�ˮ������Ϊ�Ƕ�����̼���ᵼ�¶�����̼������ƫ���������̼��������Ҳƫ�ʽ��ƫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com