
��֪��Na2CO3���Ȳ��ֽ⣬2NaHCO3 Na2CO3+CO2��+H2O��ij������Ʒ�л���������̼�����ƣ�Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ѩͬѧ��������ʵ�飺ȷ��ȡ��Ʒ10.0g�����Թ��У����ȳ�ַ�Ӧ��������ų���������CO2����224 mL(��������������ܶ�Ϊ1.964 g/L)����ش��������⣺
Na2CO3+CO2��+H2O��ij������Ʒ�л���������̼�����ƣ�Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ѩͬѧ��������ʵ�飺ȷ��ȡ��Ʒ10.0g�����Թ��У����ȳ�ַ�Ӧ��������ų���������CO2����224 mL(��������������ܶ�Ϊ1.964 g/L)����ش��������⣺
(1)����CO2������Ϊ________g(��ȷ��0.01g)��
(2)������Ʒ��Na2CO3�����������Ƕ��٣�(Ҫ��д��������̣������ȷ��0.1%)
(3)����a g�ô�����Ʒ��ּ�����������ų�����ȴ��Ƶ�ʣ�������������Ϊb g(b��a)����ô�����Ʒ��Na2CO3�����������ı��� ʽΪ_______________��(Ҫ��д���������)
ʽΪ_______________��(Ҫ��д���������)
(1)0.44 ��2���贿����Ʒ��Na2CO3������Ϊx��
2NaHCO3 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
168 44
10.0 g-x 0.44 g
 =
= x=8.32 g
x=8.32 g
������Ʒ��Na2CO3����������Ϊ ��100%=83.2%
��100%=83.2%
�𣺴�����Ʒ��Na2CO3����������Ϊ83.2%��
(3)������֪����Ӧ�������ٵ�����(a-b)gΪ���ɵ�ˮ�Ͷ�����̼������������Ʒ��̼���Ƶ���������Ϊy��
2NaHCO3 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
168 44+18
a��(1-y) (a-b)g
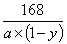 =
= ���:y=
���:y=


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ȼ�狀������Ƶ��ܽ� ��������ͼ��ʾ�����д�ͼ�л�ȡ����Ϣ����ȷ���� �� ��
��������ͼ��ʾ�����д�ͼ�л�ȡ����Ϣ����ȷ���� �� ��
A�����������ܽ�ȶ����¶��� �߶�����
�߶�����
B��45��ʱ����������Һ�����������������
C����50��������Ʊ�����Һ����20�棬ʼ��û�о�������
D��20������100g5%��������Һ������ˮʱ���Ӷ������������� Һ��������ƫ��
Һ��������ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܻ�������һ�ֹ�ҵԭ�ϣ����������˰������ӵ�����Ʒ���У��˺�������������Ʒ���������������ص�Σ������֪���ܻ���������Ҫ�ɷ����ڱ����������������ѧʽΪC24H38O4������������⣺
(1)�ڱ����������������_______(��л�������)��
(2)�ڱ����������������Է���������_______��
(3)�ڱ��������������̼���⡢������Ԫ�ص���������_______��
(4)�ڱ��������������̼Ԫ�ص�����������___________(��ȷ��0.1%)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13 gij����R���������������У�ʹ���ַ�Ӧ������RCl2�ͻ�������ų�����0.4 g����R�����ԭ��������( )
A.23 B.24 C.56 D.65
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ�ۺ����۵Ļ����7.2g��������������ַ�Ӧ���õ�����������������Ϊ( )
A.10.6g B.12.0g C.13.0g D.13.6g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��50g 98%��Ũ��������450gˮ�У�������Һ�����ʵ���������Ϊ( )
A.9.8% B.10.2% C.10.8% D.19.6%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��Ƽҵ������������°�̽�������ˡ������Ƽ���������������漰����Ҫ��ѧ��Ӧ���£�
��NH3��CO2��X NH4HCO3
NH4HCO3
��NH4HCO3��NaCl NH4Cl��NaHCO3��
NH4Cl��NaHCO3��
��2NaHCO3 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ش��������⣺
(1)��Ӧ����X�Ļ�ѧʽΪ_________��
(2)��ȥ����Na2CO3��ĩ��������NaHCO3�ķ�����_____________________��
(3)��ҵ�����к����Ȼ��ƣ�ȡ55 g��ҵ��������м���269.5 gϡ���ᣬǡ����ȫ��Ӧ������22 g������̼���ٹ�ҵ������̼���Ƶ�����������(������������0.1%)
�ڷ�Ӧ����Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��������������ȼ��ʵ��ʱ������ȼ�ĺ�������ʢ�������ļ���ƿ�У�������ȼ�գ���һ�����ȼ�ճ��ڻ���Ϩ�𡣽��������Ӽ���ƿ��ȡ��ȼ�ճף�Ϩ��ĺ����ָ�ȼ�ˡ�
��1�������������ȼ��ԭ��___________________________________________ ________________________��
________________________��
��2��������Ա�ڻ����ֳ����������Ҫ������ˮ��ԭ����____________________________________________��3.(2014������)����ֽ�۳ɺ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������ѣ�TiO2���ɾ������ڿ�����TiO2��Ti�Ļ��ϼ�Ϊ
A.��4 B. ��2 C.��1 D.��4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com