
���� ��1������ҩƷһ�����ڹ��ƿ�У�ȡ�÷�ĩ״ҩƷ��ҩ��ֽ�ۣ�
��2������ȡ�ù���ҩƷ��ע�����������
��3������ʵ�����������ҩƷ���ȵ�ʵ����������ע��������з���������Թ�ը�ѵ�ԭ������ǣ��Թܿڳ�����б���Թܵײ��Ӵ�����о������ʱ�Թ������ˮ��δ���Թ�Ԥ�ȵȣ�
��4�����ݾƾ���ʹ�õ�ע�����������
��� �⣺��1������ҩƷһ�����ڹ��ƿ�У�ȡ�÷�ĩ״ҩƷ��ҩ��ֽ�ۣ�����ͭ�̹������ڹ���Լ�ƿ�ڣ�ʵ��ʱ������ҩ��ֽ��ȡ�ã������Թ��У�
��2��ȡ�ù���ҩƷʱ����û�й涨��������ֻҪȡ�����Թܵײ����ɣ�
��3������ʱ�Թ������ˮ�����¼���ʱ�Թ����Ȳ�����ը���Թܣ��Թܵײ��Ӵ�����о�����Թ����Ȳ���ը�ѣ��Թܿ�û��������б��������ɵ�ˮ�������Թܵײ�ը���Թܣ��ռ�������Թ���������ˮ��ϴ�ȣ�
��4��Ϩ��ƾ��ƻ���ʱӦ�õ�ñ����ʵ�����ķ�Һ������Ӧ�����Һ���У�
�ʴ�Ϊ����1����ڣ�ҩ�ף�ֽ�ۣ�
��2�������Թܵײ���
��3��ABCD��
��4���õ�ñ���� ��Һ�ף�
���� ������Ҫ����ʵ�����������ҩƷ���ȵ�ʵ����������ע������ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
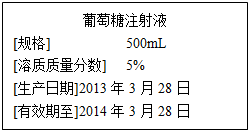 ��1�����������Ǿ������ҺA���ӱ�����ȡ������һ��ʱ�����������ʧ���γ���ҺB��������ҺA��B��һ�����ڱ���״̬���ǵ���A�������������������ϴ����B��
��1�����������Ǿ������ҺA���ӱ�����ȡ������һ��ʱ�����������ʧ���γ���ҺB��������ҺA��B��һ�����ڱ���״̬���ǵ���A�������������������ϴ����B���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ����������ȼ�շ�����ɫ���棬�ų��������� | |
| B�� | þ���ڿ����з�������ȼ�գ��������� | |
| C�� | �ⶨ��������������ʵ���У�����ȼ�ղ����������� | |
| D�� | ����������ȼ�ղ�����ɫ���棬�ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �� | C�� | �ڢ� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ������̼ | C�� | ˮ���� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 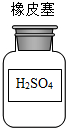 | B�� | 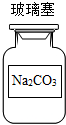 | C�� | 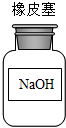 | D�� | 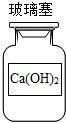 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�23gNO2����NA����ԭ�� | |
| B�� | ������1.00L•1.00mol•L-1��NaCl��Һ���ɽ�58.5gNaCl����1.00Lˮ�� | |
| C�� | ���³�ѹ�£�22.4LCCl4���и�NA��CCl4���� | |
| D�� | 1molFe��������ϡ������Һ��Ӧ��ת��3NA������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com