��ѧ��ȤС��ѧϰ�˶������̣�MnO
2�����ֽ���������ʵ���������ʵ�飮
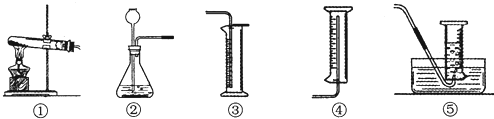
��һ��ѡ��װ�ò���������ԣ�
��������ȡ0.1�˵Ķ������̷�ĩ���������У�
��������ȡ50����������������Լ10%�Ĺ���������Һ���������У���¼һ��ʱ�����ռ��������������
���ģ��ı�������̵��������ظ�����ʵ�飬��¼�������£�
| ʱ��/�� |
�������������/���� |
| 0.1��MnO2 |
0.2��MnO2 |
0.3��MnO2 |
0.4��MnO2 |
| 40 |
49 |
61 |
75 |
86 |
| 80 |
77 |
87 |
90 |
92 |
| 120 |
89 |
92 |
92 |
92 |
| 160 |
92 |
92 |
92 |
92 |
��1��д����ʵ��Ļ�ѧ����ʽ
�����ŷ�Ӧ�Ľ��У���Һ��
H2O2
H2O2
����д��ѧʽ���ڲ��ϵؼ��١��ܼ��ڲ��ϵ�
����
����
�������ࡢ���١����䣩��
��2����ʵ��̽����������
�������̵������Թ�������ֽ������Ӱ��
�������̵������Թ�������ֽ������Ӱ��
��
��3��ʵ���г�����Ͳ�⣬����Ҫ�õ��IJ���������
��ƽ��ͣ�����������
��ƽ��ͣ�����������
��
��4��Ϊ��ɱ�ʵ�飬Ӧѡ������ͼͼ���巢�����ռ�װ���е������
�ڢ�
�ڢ�
��ѡ
����ţ�����ѡ������巢��װ�õ�������
��Ӧ���״̬�������Һ�壩�Ҳ�����
��Ӧ���״̬�������Һ�壩�Ҳ�����
��
��5����40��ʱ��ͨ���������ݷ�������ó��Ľ�����
�ڸ�ʱ���ڣ�MnO2���������࣬H2O2�ֽ���������MnO2��Ч�����ã�
�ڸ�ʱ���ڣ�MnO2���������࣬H2O2�ֽ���������MnO2��Ч�����ã�
��
��6����ʵ��ǰ����MnO
2 0.1g��ʵ����ˡ�ϴ�ӡ����������MnO
2����Ϊ
0.1
0.1
g��
�����ʵ��֤����MnO
2�Ļ�ѧ�����ڷ�Ӧǰ��û�иı�
ȡ������ͬ��������������Թ��У��������е�һ֧�Թ��м�������MnO2��ͣ�ͬʱ������֧�Թܲ����Թܵ��Ϸ����Ŵ����ǣ�ȼ�ţ���ľ������ʢ��MnO2���Թ��Ϸ���ľ���ȸ�ȼ��ȼ�յĸ����������֤��MnO2�Ļ�ѧ���ʲ���
ȡ������ͬ��������������Թ��У��������е�һ֧�Թ��м�������MnO2��ͣ�ͬʱ������֧�Թܲ����Թܵ��Ϸ����Ŵ����ǣ�ȼ�ţ���ľ������ʢ��MnO2���Թ��Ϸ���ľ���ȸ�ȼ��ȼ�յĸ����������֤��MnO2�Ļ�ѧ���ʲ���
��

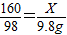 ��ã�X=16g
��ã�X=16g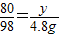 ��ã�y=4g
��ã�y=4g ×100%=8%
×100%=8%

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
