
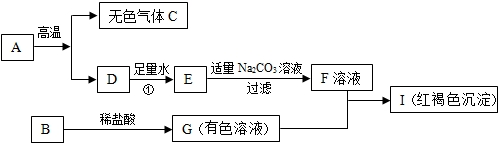
 Ä³Ę·ÅĘŃĄøą²ąĆęµÄ±źĒ©ČēĶ¼£®
Ä³Ę·ÅĘŃĄøą²ąĆęµÄ±źĒ©ČēĶ¼£®·ÖĪö £Ø1£©øł¾Ż»ÆŗĻ¼ŪŌŌņ£¬ÓÉ»ÆѧŹ½Ēó³öŌŖĖŲµÄ»ÆŗĻ¼Ū£¬øł¾Ż»ÆѧŹ½Ēó³öŌŖĖŲµÄÖŹĮæ±Č£»
£Ø2£©øł¾ŻÖøŹ¾¼ĮµÄ±äÉ«·ÖĪöČÜŅŗµÄĖį¼īŠŌ£»
£Ø3£©øł¾ŻĢ¼ĖįøłĄė×ӵļģŃé·½·Ø¼°·“Ó¦µÄĢŲµć·ÖĪö»Ų“š£»
£Ø4£©øł¾ŻŃĄøąÖŠµ„·śĮ×ĖįÄʵÄÖŹĮæ·ÖŹż·ÖĪöÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©µ„·śĮ×ĖįÄʵĻÆѧŹ½ŹĒNa2PO3F£¬ÓÉÓŚÄʵĻÆŗĻ¼ŪĪŖ+1£¬ŃõµÄ»ÆŗĻ¼ŪĪŖ-2£¬PŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+5£¬ÉčFŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖx£®£Ø+1£©”Į2+£Ø+5£©+£Ø-2 £©”Į3+x=0£¬½āµĆx=-1£¬øĆĪļÖŹÖŠNa”¢OŌŖĖŲµÄÖŹĮæ±ČĪŖ£ŗ£Ø23”Į2£©£ŗ£Ø16”Į3£©=23£ŗ24£®
£Ø2£©ŃĄøąĖ®ČÜŅŗÄÜŹ¹ĪŽÉ«·ÓĢŖ±äŗģ£¬ŌņĘä³Ź¼īŠŌ£®
£Ø3£©¼ģŃéĦ²Į¼ĮÖŠµÄŅõĄė×ÓCO32-£¬³£ÓĆ¼ÓČėĻ”ŃĪĖį£¬ŌŁ½«Éś³ÉµÄĘųĢåĶØČė³ĪĒåµÄŹÆ»ŅĖ®ÖŠĄ“¼ģŃ飬ĻČŗó·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ CaCO3+2HCl=CaCl2+H2O+CO2”ü£¬øĆ·“Ó¦µÄ»ł±¾ĄąŠĶĪŖø“·Ö½ā·“Ó¦£»CO2+Ca£ØOH£©2=CaCO3”ż+H2O£®
£Ø4£©µ„·śĮ×ĖįÄĘÖŠFŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ£ŗ$\frac{19}{23”Į2+31+16”Į3+19}$”Į100%=$\frac{19}{144}$”Į100%”Ö13.19%£»ŗ¬114mg=0.114g·śµÄµ„·śĮ×ĖįÄʵÄÖŹĮæĪŖ0.114”Ā13.19%”Ö0.864g£¬
ŃĄøąÖŠµ„·śĮ×ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ$\frac{0.864g}{110g}$”Į100%”Ö0.79%£¬
¼ĘĖć½į¹ūĪŖ0.79%ŌŚ0.76%ŗĶ0.80%Ö®¼ä£¬¾ßÓŠ½ĻŗƵķĄČ£³ŻŠ§¹ū£®
¹Ź“šĪŖ£ŗ£Ø1£©-1£¬23£ŗ24£»£Ø2£©¼īŠŌ£»£Ø3£©CaCO3+2HCl=CaCl2+H2O+CO2”ü£¬ø“·Ö½ā£¬CO2+Ca£ØOH£©2=CaCO3”ż+H2O£»£Ø4£©¾ßÓŠ£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖ»ÆѧÓĆÓļµÄŹéŠ“”¢ŅŌ¼°øł¾Ż»ÆѧŹ½µÄ¼ĘĖćµČ£¬ŹōÓŚæĪ±¾µÄ»ł“”ÖŖŹ¶£¬Ó¦¼ÓĒæѧĻ°£®


 Š”ѧʌĩ³å“Ģ100·ÖĻµĮŠ“š°ø
Š”ѧʌĩ³å“Ģ100·ÖĻµĮŠ“š°ø ĘŚÄ©ø“Ļ°¼ģ²āĻµĮŠ“š°ø
ĘŚÄ©ø“Ļ°¼ģ²āĻµĮŠ“š°ø ³¬ÄÜѧµäµ„ŌŖĘŚÖŠĘŚÄ©×ØĢā³å“Ģ100·ÖĻµĮŠ“š°ø
³¬ÄÜѧµäµ„ŌŖĘŚÖŠĘŚÄ©×ØĢā³å“Ģ100·ÖĻµĮŠ“š°ø »ĘøŌ360¶Č¶ØÖĘĆܾķĻµĮŠ“š°ø
»ĘøŌ360¶Č¶ØÖĘĆܾķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĒāĘų | B£® | ľĢæ | C£® | Ć¾“ų | D£® | Įņ·Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Īļ ÖŹ | Ń”ÓĆŹŌ¼Į | Ö÷ŅŖ²Ł×÷ | |
| A | CO2£ØCO£© | O2 | µćČ¼ |
| B | MnO2£ØKCl£© | H2O | ¹żĀĖ |
| C | NaClČÜŅŗ£ØCuCl2£© | AgNO3ČÜŅŗ | ½į¾§ |
| D | Fe£ØCu£© | H2SO4 | ¹żĀĖ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | æÕĘųÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲŹĒµŖŌŖĖŲ | |
| B£® | µŲæĒÖŠÖŹĮæ·ÖŹż×ī“óµÄ½šŹōŌŖĖŲŹĒĢśŌŖĖŲ | |
| C£® | ĖįÖŠŗ¬ĒāŌŖĖŲ | |
| D£® | ¼īÖŠŗ¬Ēā”¢ŃõŌŖĖŲ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ī÷øńĮŠĶ”µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ407g | |
| B£® | Ī÷øńĮŠĶ”ÖŠĢ¼ŌŖĖŲÖŹĮæ·ÖŹż×īøß | |
| C£® | Ī÷øńĮŠĶ”ÖŠÓŠ43øöŌ×Ó | |
| D£® | Ī÷øńĮŠĶ”ÖŠµŖ”¢ŃõŌŖĖŲµÄÖŹĮæ±ČĪŖ5©s1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com