
��7�֣�ijͬѧ��������ʵ�飺

ʵ�����ݼ�����ʵ���������±���
| ��һ�� | �ڶ��� |
������ͭ��������g�� | m | m |
��ϡ�����������g�� | 50 | 100 |
������������Һ��������g�� | 100 | 100 |
B����Ҫ���� | ����ɫ���� | �� |
����һ������ÿһ����ǡ����ȫ��Ӧ����������������Һ�����ʵ���������Ϊ8��55%����ش��������⣺
1��д����һ����������Һ��ɫ�ı�Ļ�ѧ��Ӧ����ʽ ��
2���ڶ���ʵ��B�е���Ҫ������ ��
3�������м�������ͭm����ֵΪ ��
4�����ڶ��η�Ӧ�����ɹ���������X���ı���ʽ ��
5�������ڶ��η�Ӧ�����Һ����32��35��ˮ�������ò�������Һ�����ʵ���������Ϊ ;
6������98%��Ũ���������������������ᣬ����Ҫ��ˮ������Ϊ ��
(1) CuO + H2SO4 = CuSO4 + H2O Ba(OH)2 +CuSO4 =Cu(OH)2�� + BaSO4��
(2)������ɫ����(��ɫ��Һ��dz) (3) 4
(4) 171��233=8��55g/X ��5��5% ��6��135g
��������
���������(1) ��һ��������������ͭ�м���ϡ���ᣬ������Ӧ��������ͭ��ɫ��Һ����ѧ��Ӧ����ʽΪ ��CuO + H2SO4 = CuSO4 + H2O�����ɵ�����ͭ��Һ����������������Һ��Ӧ����ѧ��Ӧ����ʽΪ ��Ba(OH)2 +CuSO4 =Cu(OH)2�� + BaSO4��
(2) ���ڵ�һ������ÿһ����ǡ����ȫ��Ӧ�����Եڶ���ʵ����� 100gϡ���ᣬ˵�����������Եڶ���ʵ��B�е���Ҫ������������ɫ����(��ɫ��Һ��dz)
(3)���ݻ�ѧ��Ӧ��CuO + H2SO4 = CuSO4 + H2O��Ba(OH)2 +CuSO4 =Cu(OH)2�� + BaSO4�������Կ���CuO��Ba(OH)2��������ϵ80��171������������������=100g��8��55%=8��55g�����Բ������CuO������m=4g
��4���ڶ��η�Ӧ�����ķ���ʽΪ��Ba(OH)2+H2SO4==BaSO4��+2H2O����Ba(OH)2��BaSO4��������ϵΪ171:233����Ba(OH)2������Ϊ100g��8��55%=8��55g�����Կ���BaSO4������Ϊx���ʿ���ʽΪ��171��233=8��55g/X
��5���ڶ��η�Ӧ�����Һ�Ǽ�����100gϡ���ᷴӦ�õ�����Һ �����ݵ�һ�η�Ӧ��������ϵ��CuO + H2SO4 = CuSO4 + H2O���ɼ��������CuSO4������Ϊ 8g������������ʽ��Ba(OH)2+H2SO4==BaSO4��+2H2O�������BaSO4������=11��65g������Һ����=4g+100g+100g-11��65g=192��35g,���������ڶ��η�Ӧ�����Һ����32��35��ˮ����ʱ��Һ��������Ϊ=192��35-32��35g=160g�������ò�������Һ�����ʵ���������=8g/160g��100%=5%
��6�����ݷ�ӦCuO + H2SO4 = CuSO4 + H2O��CuO��H2SO4��������ϵΪ80:98��CuO������m=4g������H2SO4������=4��9g������H2SO4����������=4��9g/50g��100%=9��8%�������ĵ�ϡ����������Ϊ150g�����Ը���ϡ��ǰ��������������䣬����98%��Ũ��������Ϊ x������ʽΪ ��98%��x=150g��9��8%,����x=15g,��ˮ������=150g-15g=135g
���㣺��ѧ��Ӧ����ʽ����д�����ݻ�ѧ��Ӧ����ʽ���㣬��Һ��ϡ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�п���ʦ�Ƽ���ѧ--��ѧ̽�� ���ͣ�̽����
ij��ѧ��ȤС���������ͼ��ʾ��װ�ã��Դ��Na2CO3����С�մ�NaHCO3�����ֹ������ʵ��̽��
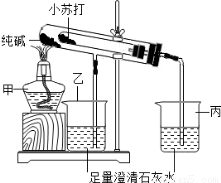
��1��д���ס����������������ƣ�_________��_________
��2����ʵ������У��۲쵽�������еij���ʯ��ˮ����ǣ���д���������з�����Ӧ�Ļ�ѧ����ʽ____________________________��
��3����ʵ��̽�����õĿ�ѧ������______________���������Ʊ������������Աȹ۲취����������������������ʵ��Ŀ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�п���ʦ�Ƽ���ѧ--��Ϣ�������ͼ���� ���ͣ�ѡ����
�����»�ѧʵ�����й�ҩƷ��ͼ���У����ڸ�ʴƷͼ���ǡ�



A B C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�ϲ��Զ���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ճ������е�������������������-------------------------------------------( )
A���ô������ϴ�Ӽ�ϴ�� B�����������ƴ���ʳ�ε�ζ
C�����Ż�ǹ��� D���ó涣ҧ��ͿһЩ���������ʵ���Һֹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�ϲ��Զ���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ԭ�ӽṹʾ��ͼ����ʾ��Ԫ�ػ�ѧ�������ȶ�����--------------------------( )
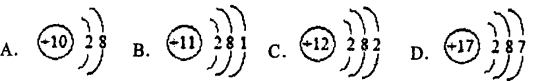
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�������е������п����в��Զ���ѧ�Ծ��������棩 ���ͣ������
��3�֣��������������ľ�����������������Ļ��������ѳ��Ѿ�����ü�ޡ������Ҫ��ش����⣺

����β����������������� ��Ϊ�˼��������������⣬Ŀǰ�����ǹ�������������ʹ�õ����Ҵ����ͣ�����̸һ̸ʹ���Ҵ����͵��ŵ���______��Ϊ�˽�һ����������������������������������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�������е������п����в��Զ���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й���Դ��Դ��������ȷ���ǣ� ��
A������Դ�Ŀ��������ú���Ҫ����������Ӧ�ô����������ܣ����ܣ����ܵ�����Դ��
B���ҹ��Ľ�����رȽϷḻ������ͭ�������Ƚ������Ե��ʵ���ʽ����
C���Ȼ������˵��������ʮ����Ҫ���ҹ����Ȼ�����Դ���ں�����
D��������Χ�Ŀ����ڹ�ũҵ�����п������ڻ��ʣ����֣�ʯ�ͼӹ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ���������ɱ����п����в��Զ���ѧ�Ծ��������棩 ���ͣ������
(2��)���ɷ�����ѧϰ��ѧ����Ҫ�����������������ɵIJ�����������ʽ��з��ࣺ
Na2CO3��Na2SO4��NaCl��K2CO3��BaSO4��CaCO3��
��1��ѡ����һ������Ϊ______��
��2������������________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��������ƽ�����п���ģ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���й������彡����������ȷ����������������������������������������( )
A��������θҺ��pHֵΪ1.5��1.9
B��п����Ԫ�أ�ȱп����ƶѪ��п����Ҫʳ����Դ�к���Ʒ�����⡢�����
C����ֹ����Ǧ���ӵ��к�Ԫ�ض����彡�����ֺ��ǽ����������Ҫ��֤
D��������������ζ�����ʳ����������ܴٽ����彡��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com