
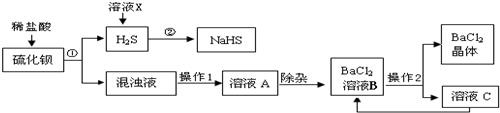
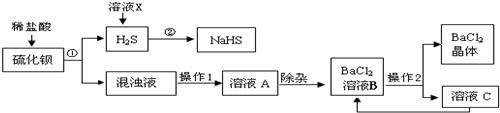
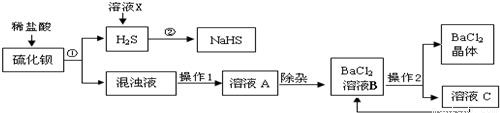
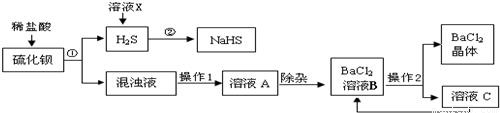
��ҵ�Ͽ���ú��ԭ�ؾ�ʯ�������Ʊ�BaCl2���������̼�ͼ���£�
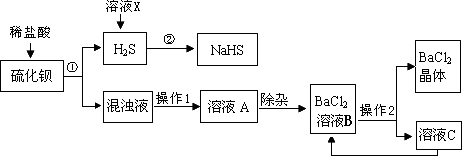
(1)���Ļ�ѧʽΪ________������ϡ���ᷢ�����ֽⷴӦ�Ļ�ѧ����ʽΪ________(��ʾ������H2S����)��
(2)����1��________������2�ǽ���ҺB����Ũ����________�����˵ȣ�
(3)���ᱵ�׳Ʊ��ͣ��������ڼ��θ�������ɽ�̼�ᱵ�������ᱵ��ԭ����________��
(4)H2S�����ж���ˮ��Һ��ʹ��ɫʯ�����________�ԣ�������ҺX��H2Sת��ΪNaHS��H2O������ҺX��������________����Ӧ�Ļ�ѧ����ʽΪ________��
(5)����������ѭ�����õ�������________����������Ŀ����________��
 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�������������У�ѧ����У�����꼶���£���ʮ������ĩ��ҵ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ�����б�Ӧ���п���ѧģ���Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com