
ͬѧ�Ǵ�ɽ�ϲɼ���һ��ʯ��ʯ(��Ҫ�ɷ�Ϊ̼���),����ȡ80 g����Ʒ��������ʵ��(�������������չ����в������仯),��÷�Ӧ�����������뷴Ӧʱ��Ĺ�ϵ���±�:
| ��Ӧʱ��/s | t0 | t1 | t2 | t3 | t4 | t5 | t6 |
| ��Ӧ������ ����/g | 80 | 75 | 70 | 66 | 62 | 58 | 58 |
��ش���������:
(1)��ʯ��ʯ��ȫ��Ӧ��,����CO2������Ϊ ��__________��
��__________��
(2)���ʯ��ʯ�к�CaCO3����������(д���������)��
������������ͼ�б����֪��t5ʱ�����������ټ���,��֪̼�����ȫ�ֽ�,���������غ㶨�����ɶ�����̼������Ϊ80 g-58 g=22 g,����̼��Ʒֽ�Ļ�ѧ����ʽ,��̼����������̼��������ϵ,���ݶ�����̼���������Լ���̼��Ƶ�����,�Ӷ����Լ���̼��Ƶ�����������
��:(1)22 g
(2)����Ʒ�к�CaCO3������Ϊx
CaCO3 CaO+CO2��
CaO+CO2��
100 44
x 2 2 g
2 g
 =
=
x= =50 g
=50 g
��Ʒ��CaCO3����������= ��100%=62.5%
��100%=62.5%
��:��Ʒ��CaCO3����������Ϊ62.5%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й�ȼ�ϵ�����˵��,����ȷ���ǡ�(����)
A.��ʯȼ�϶��ǿ���������Դ
B.����������Ľྻ������ȼ��
C.ʯ�����ɲ�ͬ��������ɵĻ����
D.úȼ��ʱ����������������Ⱦ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�����,�����й��������˺���ɴ����÷�Ӧ2Na2O2+2CO2 2R+O2���ṩ����,����R�Ļ�ѧʽ�ǡ�(����)
2R+O2���ṩ����,����R�Ļ�ѧʽ�ǡ�(����)
A.CO���� B.Na2O���� C.NaOH���� D.Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ҫ��,д���йػ�ѧ��Ӧ�Ļ�ѧ����ʽ��
(1)�к�ɫ�������ɵĻ��Ϸ�Ӧ:
 ����
����
(2)ʵ������һ�ִ�������ȡ����:
����
(3)���۴�����װ�IJ�������,����Һ�嶡��(C4H10),�����ڿ�����ȼ�պ����ɶ�����̼��ˮ����,д��������ȫȼ�յĻ�ѧ����ʽ:��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ɫ��̼�����ϵ�����ɫʯ����Һ��,Ȼ���ټ���,��Һ��ɫ�ı仯�����(����)
A.�ȱ�������� B.������ɫ���ٸı�
C.�ȱ���ɫ���� D.�ȱ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ���ڽ��ж�����̼����ȡ������ʵ��ʱ,������������о�,�Կα��е����巢��װ��(��ͼA)�����˸Ľ�(��ͼB)��

(1)�Ľ���װ�õ��ŵ���___________________________________________��
(2)���ƵõĶ�����̼����ͨ��ʢ�г���ʯ��ˮ���Թ���,�۲쵽��������
_____________________________________________ ___________________��
___________________��
(3)��D�е�CO2��C�ձ��Ҳ൹��������ʵ��ʱ,�۲쵽��������_________
__________________________________________________________________��
(4)Ϊ��̽��H2CO3��������,��CO2����������,��ͬѧ���������ʵ��(��ͼ)����д���۲쵽������:

�������� ����:��ֽ________�������� ����:��ֽ______
��:����Ϊ��ͨ������ʵ��________(��ܡ����ܡ�)�ﵽʵ��Ŀ�ġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڶ�����̼����ʶ��,��ȷ��һ����(����)
�ٶ�����̼��һ����̼��һ����ԭ��
�ڶ�����̼�ɹ���ɫֲ��������
�۾�δ�����IJ˽Ѷ�����̼�ĺ����ȿ����еĸ�
�ܶ�����̼��ʹ��ʯ����ҺȾ����ɫ�ĸ���ֽ�����
A.�٢ڢۡ��� B.�ڢۡ����� C.�ڢۢܡ��� D.�٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ���ӷֽ�ʾ��ͼ�������ܻ��������Ϣ������������������
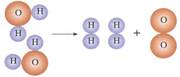 A���ڻ�ѧ�仯��ԭ������С������
A���ڻ�ѧ�仯��ԭ������С������
B��������ܱ��������Ļ�ѧ����
C��ˮ�ֽ�ʱԪ�ص������
D������һ�����Ϸ�Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com