
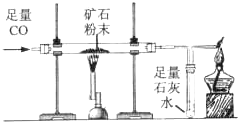
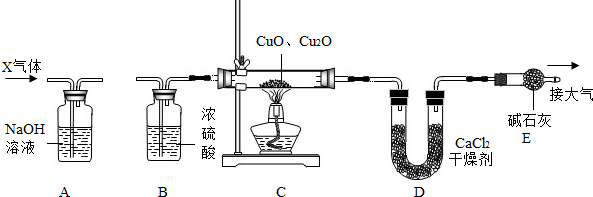
 ĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶Øijŗ¬ĮāĢśæóµÄæóŹÆѳʷ֊Ģ¼ĖįŃĒĢśµÄÖŹĮæ·ÖŹż£ØŌÓÖŹ²»ŗ¬ĢśŌŖĖŲĒŅŌŚŹµŃé¹ż³ĢÖŠ²»·¢ÉśČĪŗĪ±ä»Æ£©£¬ŹµŃ鏿¾Ż¼ĒĀ¼ŌŚĻĀ±ķÖŠ£®
ĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶Øijŗ¬ĮāĢśæóµÄæóŹÆѳʷ֊Ģ¼ĖįŃĒĢśµÄÖŹĮæ·ÖŹż£ØŌÓÖŹ²»ŗ¬ĢśŌŖĖŲĒŅŌŚŹµŃé¹ż³ĢÖŠ²»·¢ÉśČĪŗĪ±ä»Æ£©£¬ŹµŃ鏿¾Ż¼ĒĀ¼ŌŚĻĀ±ķÖŠ£® ””””FeO+CO2”ü
””””FeO+CO2”ü| ŹµŃéĒ° | ŹµŃéŗó | |
| Ó²ÖŹ²£Į§¹Ü£Øŗ¬ŃłĘ·£© | ””””165.6g | ””””159.6g |
| Ėµ””Ć÷ | ¢ŁæÕÓ²ÖŹ²£Į§¹ÜÖŹĮæĪŖl45.6g ¢Śŗ¬Ģśø÷ĪļÖŹ·“Ó¦ĶźČ« | |
 FeO+CO2”ü£¬CO+FeO
FeO+CO2”ü£¬CO+FeO Fe+CO2”ü£¬CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O£®
Fe+CO2”ü£¬CO2+Ca£ØOH£©2ØTCaCO3”ż+H2O£®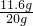 ”Į100%=58%
”Į100%=58%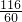 =
=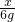
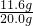 ”Į100%=58%
”Į100%=58%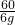 =
=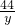 =
=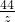



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶Øijŗ¬ĮāĢśæóµÄæóŹÆѳʷ֊Ģ¼ĖįŃĒĢśµÄÖŹĮæ·ÖŹż£ØŌÓÖŹ²»ŗ¬ĢśŌŖĖŲĒŅŌŚŹµŃé¹ż³ĢÖŠ²»·¢ÉśČĪŗĪ±ä»Æ£©£¬ŹµŃ鏿¾Ż¼ĒĀ¼ŌŚĻĀ±ķÖŠ£®
ĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶Øijŗ¬ĮāĢśæóµÄæóŹÆѳʷ֊Ģ¼ĖįŃĒĢśµÄÖŹĮæ·ÖŹż£ØŌÓÖŹ²»ŗ¬ĢśŌŖĖŲĒŅŌŚŹµŃé¹ż³ĢÖŠ²»·¢ÉśČĪŗĪ±ä»Æ£©£¬ŹµŃ鏿¾Ż¼ĒĀ¼ŌŚĻĀ±ķÖŠ£®
| ||
| ŹµŃéĒ° | ŹµŃéŗó | |
| Ó²ÖŹ²£Į§¹Ü£Øŗ¬ŃłĘ·£© | 165.6g | 159.6g |
| Ėµ Ć÷ | ¢ŁæÕÓ²ÖŹ²£Į§¹ÜÖŹĮæĪŖl45.6g ¢Śŗ¬Ģśø÷ĪļÖŹ·“Ó¦ĶźČ« | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
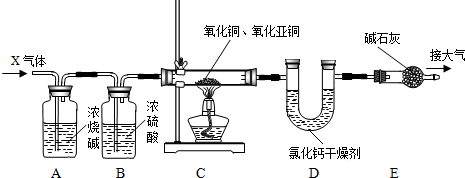
 £Ø2012?Īäŗŗ£©Ä³»ÆѧŠ”×éĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶ØĶŠæŗĻ½šŃłĘ·ÖŠŠæµÄÖŹĮæ·ÖŹż£ØĶ¼ÖŠ¹Ģ¶Ø×°ÖĆŅŃĀŌČ„£©
£Ø2012?Īäŗŗ£©Ä³»ÆѧŠ”×éĄūÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ²ā¶ØĶŠæŗĻ½šŃłĘ·ÖŠŠæµÄÖŹĮæ·ÖŹż£ØĶ¼ÖŠ¹Ģ¶Ø×°ÖĆŅŃĀŌČ„£©²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com