
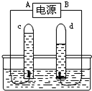
ijͬѧΪ�ⶨ�����������ĺ������������ͼ��ʾ��ʵ��װ�á���ͬѧ�ڡ������ݡ���ÿһ���İ�������һ������ֽ����ˮ��İ��ף��÷Ŵ��6V�ֵ�Ͳ���ڿ���ˮ���һ���������ݡ����İ����ϡ�
��1��һ��ʱ��ɹ۲쵽����Ҫ������ ��
��2���������ݡ���ÿһ���϶�����һС�Ű�����ֻ����ˮ���һ���������ݡ�����һ��Ű�����ȣ��ŵ��� ��
��3��д������ѧ���IJ�����ʵ������ķ�Ӧ�Ļ�ѧ����ʽ�� ��
��1����������¥�ݡ�������ȼ�գ���ʼʱð���̣�ȼ�տ�ֹͣʱ�ڵ����Թܵ��ϲ����ֻ��̡����ڰ���ȼ���������Թ��ڵ�������ʹ�Թ��ڵ�ˮλ������һ��λ�ú�㶨����
��2�������ܶ�������Թ��ڵ�����
��3��4P��5O2��2P2O5
�������������(1)�����Ż��ͣ��÷Ŵ��6V�ֵ�Ͳ���ڿ���ˮ���һ���������ݡ����İ����ϣ�����������¥�ݡ�������ȼ�գ���ʼʱð���̣�ȼ�տ�ֹͣʱ�ڵ����Թܵ��ϲ����ֻ��̡��Թ��ڵ�ˮλ������һ��λ�ú�㶨����
(2) �������ݡ���ÿһ���϶�����һС�Ű�����ֻ����ˮ���һ���������ݡ�����һ��Ű�����ȣ��ŵ��Ǿ����ܶ�������Թ��ڵ�����
(3) ����ȼ�շ�Ӧ�Ļ�ѧ����ʽ��4P��5O2��2P2O5
���㣺���������������IJⶨ


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣�ˮ����Һ������������������ʮ����Ҫ�����á�
��1����ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�1������������ ��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ ��
��2����Դˮ����������ˮ�Ĺ������������ʯ�ң���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ__ __��
��3��20��ʱ���Ȼ��Ƶ��ܽ��Ϊ36g����20��ʱ�Ȼ��Ʊ�����Һ�����ʺ��ܼ���������Ϊ ��
��4��Ϊ�˽���ũҵѡ�֣��ֽ�200g30%���Ȼ�����Һϡ��Ϊ10%���Ȼ�����Һ����Ҫ��ˮ������Ϊ ��
��5������ˮ��ͨ��������������ɱ������������������Ũ��ˮ������������豸�����������Ƿ�������й©��A��B��C��D��ʾ4�����ʣ�����ʾ��ͼ���±���A��B��һ�������·�Ӧ����C��D��
| ���� | A | B | C | D |  |
| ��ѧʽ | NH3 | Cl2 | N2 | | |
| ��ʾ��ͼ |  |  |  |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013 �������������ͻȪ��������������̶�����Ǻӵ�������Դ���齨���µ�һȪ�羰�����������ص���Ȼɽˮ���ۺ�������ʷ�Ļ�������һ�壬Ϊ 5A ��������
��1����ͻȪˮ����__________����������������
��2��ij��ѧС���ͬѧ�Ի��Ǻӵ�ˮ����������ص��о�����Ҫ�� pH ��ֽ���Բⶨ��ˮ�������ǿ�����ⶨ�ľ��巽����
��3��Ϊ�˼��������ˮ����ˮ����Ӳˮ������ˮ���м���___________�����衣
��4��������Ȫˮ֮������Ȫˮ������˵���д������ ������ţ�
| A���峺��Ȫˮ����Һ | B�������Ǿ����̶���ߵľ�ˮ���� |
| C��������еķ�������Ȫˮ��Ӳ�� | D�����˿��Գ�ȥȪˮ�еĿ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ˮ��һ����������������ģ�����Ӧ���˽��й�ˮ��һЩ֪ʶ��
��1������ˮ�������䰮ˮ��Դ����ÿ������Ӧ�������κ������������������ڱ���ˮ��Դ���� (�����)��
A������ʹ�û���ũҩ B����ҵ��ˮ���������ŷ�
C��ʹ�ú���ϴ�·� D��������ˮֱ���ŷ�
��2��ˮҲ��������ܼ������������ʼ���������ˮ�У����γ���ɫ��Һ���� ��������ĸ��ţ�
A��ֲ���� B������ C��CaCO3 D���������
��3���ܽ��˽϶�ĵĿ����Ըƺ�þ�Ļ������ˮ����Ӳˮ��Ӳˮ������������в���Ӱ�졣�����У�һ����� �ķ�����ʹӲˮת��Ϊ��ˮ��
��4���ҹ����Ƴ���Ư�۸���Ч������ˮ��������ClO2������ȡClO2��ӦΪ��
X + 2NaClO2 ="=" 2ClO2 + 2NaCl����X�Ļ�ѧʽΪ ��
��5��д��һ����ˮ�μӵĻ��Ϸ�Ӧ�Ļ�ѧ��Ӧ����ʽ�� ��
��6����ʵ������Ũ��������ϡ����ʱ����Ҫ�õ�ˮ������Ҫ�����У����㡢 �����ȡ���ȴ������װƿ�����ϱ�ǩ�� ��Ҫ����184g��������Ϊ10%��ϡ���ᣬ��Ҫ��������Ϊ98%��Ũ����(�ܶ�Ϊ1.84 g/cm3) mL(����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����벻��ˮ������ˮ������ͨ�������������� �����þ�ˮ����װ�л���̿������Ϊ������ �ԡ�ͼһ�ǵ��ˮ��ʵ��װ�ã���ԴA���� �����˷�Ӧ�Ļ�ѧ����ʽΪ ��ͼ����ˮ������ʾ��ͼ������ȷ��ʾ��Ӧ���̵�˳���� ������Ԫ��������̬���ڵ�ͼʾ�� ������дͼʾ��ţ���ʵ����Եó����½��ۣ� ��
 |  | ||||
| ͼһ | ͼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������������,�����ǵ�����ϢϢ��أ���ش����������еĻ�ѧ���⡣
��1������̼�����ʽ�еġ���̼��ָ����̼______(�� �����ʡ���ԭ�ӡ���Ԫ�ء�)���ճ���������ġ���̼��������� ������һ����
��2�� �豭�ڵ�ɴ���ɽ���Ҷ���ˮ����������ã������ԭ�����ƻ�ѧʵ������е�___ _���ڼ����ݲ�ʱ��Ҫ��֪��������ˮ��Ӳˮ������ˮ�ķ����� ��
��3�����ʵĽṹ�������ʵ����ʺ���;���ڼ�ͥú¯ȼ��úʱ���ѳɷ���ú����������ԭ���� ���ù��ķ�����ú��ŵ���ˮ����,������ȥ����ζ��ɫ�ص�Ч����������ú��������뾻ˮ���� __����(���й����ʼ��������)��Ϊ�˼���Դ�������Ⱦ���ö��ͥ�����˸�Ϊ��������ȼ�ϡ�����Ȼ������д����Ȼ����Ҫ�ɷ�ȼ�յĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС���ͬѧ�������ͼ��ʾ�ļ��ĵ��ˮʵ��װ�ã����м�ͥСʵ�飬������������ݳ�������������±���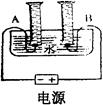
| ʱ�� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ���ӵ�Դ��������ͲA������ | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 |
| ���ӵ�Դ��������ͲB������ | 2 | 4 | 6 | 9 | 12 | 15 | 18 | 21 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��д����ѧ����ʽ���ش��й����⣺
��1��ˮ��ͨ��ʱ������⣺��_ ________����
�ڸ�ʵ���У��ɿ��������븺������������������_________������ʵ��ó��Ľ�����ˮ����____ _____��ɡ�
��2��ϸ��˿�ڴ�������ȼ�գ���__ _______����
��ʵ����Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ��ԭ������___ __ ____ ����
��3����ͼ��ͼ�к���ȼ�յĻ�ѧ����ʽΪ��_______ __ ����
��ֹˮ�к�����������_______ __��˵��������������Լռ_ ___������ͨ��ʵ��ó�������۵Ļ�ѧ����__ __������ţ���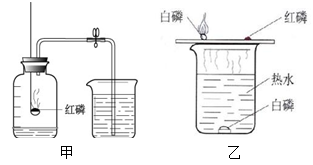
| A�������� | B�������� | C�������� | D����ķ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ĿǰΪ�ֿ����ϴ���������֯��������������ȡ����ˮ�������նɹ��ѹأ��������Ⱦ��Ӳ�ȴ�ĵ���ˮ���������彡����Ϊ�����ǻ�Ӧ������н�һ���Ĵ�����
��1���ⶨ����ˮ�����ȣ��������� ����
��2������ˮ������������������������������ͨ��ʹ��Ư�ۣ�����Ч�ɷִ�����ƿɷ������·�Ӧ��Ca��ClO��2+X+H2O=CaCO3��+2HClO����X�Ļ�ѧʽΪ�� ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com