
 ij��ѧ��ȤС���ͬѧ��12gʯ��ʯ��Ʒ��100gϡ���ᣨ�����������ձ��У��ڻ�ѧ��Ӧ�����ж��ձ�������ʣ����������γ�������¼������������ʲ�����ˮ��Ҳ����ϡ���ᷴӦ���ձ�������Ϊ25g��
ij��ѧ��ȤС���ͬѧ��12gʯ��ʯ��Ʒ��100gϡ���ᣨ�����������ձ��У��ڻ�ѧ��Ӧ�����ж��ձ�������ʣ����������γ�������¼������������ʲ�����ˮ��Ҳ����ϡ���ᷴӦ���ձ�������Ϊ25g��| ��Ӧʱ�䣨s�� | t0 | t1 | t2 | t3 | t4 | t5 |
| �ձ���ҩƷ������g�� | 137 | 135 | 133.5 | 133 | 132.6 | 132.6 |
���� ���������غ㶨�ɿ�֪�������������ļ�������Ϊ�����˶�����̼�����Կ������������̼�����������ݶ�����̼�������Ͷ�Ӧ�Ļ�ѧ����ʽ����̼��ƺ��Ȼ��Ƶ����������������Ӧ������������
��� �⣺���������غ㶨�ɣ�������̼������Ϊ��137g-132.6g=4.4g
���ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 111 44
x y 4.4g
$\frac{100}{x}$=$\frac{111}{y}$=$\frac{44}{4.4g}$
x=10g
y=11.1g
��Ӧ��ȫ��������Һ���Ȼ��Ƶ�������������Ϊ$\frac{11.1g}{10g+100g-4.4g}$��100%��10.5%
��3�������ձ������ʵ��������仯���������ɵĶ�����̼�����������Կɵ����±���
| ��Ӧʱ�䣨s�� | t0 | t1 | t2 | t3 | t4 | t5 |
| �ձ���ҩƷ������g�� | 137 | 135 | 133.5 | 133 | 132.6 | 132.6 |
| �����仯����������̼������ | 0 | 2 | 3.5 | 4 | 4.4 | 4.4 |

 ��
������ ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������Ͻ����ճ�������õĽ������ϣ���;�dz��㷺����ش��������⣺
������Ͻ����ճ�������õĽ������ϣ���;�dz��㷺����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ��ȥ���ʵķ��� |
| A | FeSO4��Һ��H2SO4�� | �����������ۣ���ַ�Ӧ����� |
| B | CO2��CO�� | ͨ���������������ȼ |
| C | H2��HCl�� | ��ͨ��������NaOH��Һ����ͨ��������Ũ���� |
| D | KCl��KClO3�� | ���������ٲ�������Ϊֹ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 14��11 | B�� | 7��11 | C�� | 11��14 | D�� | 11��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
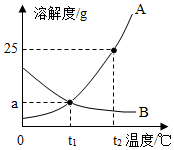 ��ͼΪA��B���ֹ������ʵ��ܽ�����ߣ�
��ͼΪA��B���ֹ������ʵ��ܽ�����ߣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ռ����ڼ� | B�� | ���ϡ���ë���ںϳɲ��� | ||
| C�� | ���顢�ƾ������л��� | D�� | ˮ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
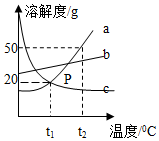 ��ͼ��a��b��c�������ʵ��ܽ�����ߣ�a��c�ܽ�����߽���P�㣬��ͼ�ش����⣺
��ͼ��a��b��c�������ʵ��ܽ�����ߣ�a��c�ܽ�����߽���P�㣬��ͼ�ش����⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com