



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������п����� ���ͣ������
 CO+H2 ��CO+H2O==CO2+H2
CO+H2 ��CO+H2O==CO2+H2

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
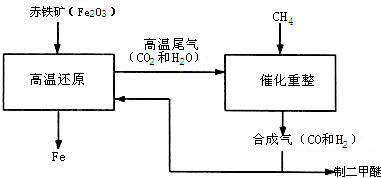
һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ġ�
��1��ú��������Ҫ��Ӧ�У���2C+O2=2CO ��C+H2O=CO+H2 ��CO+H2O=CO2+H2
������Ӧ���ڻ��Ϸ�Ӧ���� ������ţ�������������Ӧ���� ������ţ���
��2���ϳ�����ͨ����Ȼ���������õ�����CH4+H2O=CO+3H2 �ϳ������ƶ����ѣ������ѱ���Ϊ21���͵�����ȼ�ϡ��ϳ�����������ұ������������ұ�����IJ�����������ʾ�����£�

�ٶ����ѣ�CH3OCH3�����ɺϳ�����CO��H2����һ�����������Ƶġ��úϳ����ƶ�����ʱ����������һ�ֿɲ������ѭ���ġ�����ΪҺ̬�������д���÷�Ӧ�Ļ�ѧ����ʽ��
��
�ںϳ�����ұ��������������������������� ��
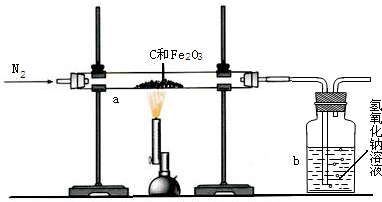
��3����ѧ��ȤС���ͬѧΪ�˲ⶨij������ʯ��������������������
��ͬѧȡһ�������ij�������������ľ̿�ۻ�Ϻ�����ͼ��ʾװ���Ժ����IJ�������ⶨ(��������ʼ�ղ������仯)��

��ʵ���г���ͨ�����ĵ���������ǰ����ͨ��һ��ʱ�䣬�������� ��
��ֹͣ����ǰ�Ƿ���Ҫ�ȶϿ�a��b�����Ӵ��Է�ֹ������Ϊʲô��
��
���������������Һ�Զ�����̼����������ȫ�ģ���ô��ͬѧ������������Һ������仯�ⶨ�������������������� (ѡ�ƫ����ƫС����ȷ��)��ԭ���� ��
����ͬѧȡ��ʯ��Ʒ10g����������ϡ���ᣬ��ȫ��Ӧ����ȥϡ����109��5g���˵õ�����2 g(�������ʼȲ�����ˮҲ�����ᷢ����Ӧ)���������ͬѧ—����������ʯ�������������������ͷ�Ӧ����Һ�����ʵ���������������д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ĵ�ʡüɽ������������ѧУ�п���ѧ��ģ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ�Ͼ����п���ѧ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com