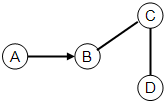
 A��B��C��D��Ϊ���л�ѧ�еij������ʣ�������֮��Ĺ�ϵ��ͼ��ʾ��ͼ�С�-����ʾ������������һ�������¿��Է�Ӧ����������ʾ���ʴ���ת����ϵ����A��B��C��Ϊ�����A��B������ͬԪ�أ�B��������ܼ����ش��������⣺
A��B��C��D��Ϊ���л�ѧ�еij������ʣ�������֮��Ĺ�ϵ��ͼ��ʾ��ͼ�С�-����ʾ������������һ�������¿��Է�Ӧ����������ʾ���ʴ���ת����ϵ����A��B��C��Ϊ�����A��B������ͬԪ�أ�B��������ܼ����ش��������⣺
��1������A�Ļ�ѧʽ______��
��2��B��C��Ӧ�Ļ�������______��
��3������C���׳�______��
��4��д��C��D����ܷ�����Ӧ�Ļ�ѧ����ʽ��д��һ�����ɣ���______��
�⣻A��B������ͬԪ�أ�B����õ��ܼ�������B����ˮ��A����˫��ˮ��C�������C��ˮ�ᷴӦ������C�����dz����������ƣ������ƻ���ᷴӦ����ˣ�
��1������A�ǹ������⣬���Ļ�ѧʽΪ��H2O2��
��2��B��C��Ӧ��ˮ�������Ʒ�Ӧ�����������ƣ����ڻ��Ϸ�Ӧ��
��3�����������Ƶ��׳�����ʯ�ң�
��4��C��D��ķ�Ӧ��������ʯ��������ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CaO+2HCl=CaCl2+H2O��
�ʴ�Ϊ����1��H2O2����2�����Ϸ�Ӧ����3����ʯ�ң���4��CaO+2HCl=CaCl2+H2O��
����������A��B������ͬԪ�أ�B����õ��ܼ�������B����ˮ��A����˫��ˮ��C�������C��ˮ�ᷴӦ������C�����dz����������ƣ������ƻ���ᷴӦ��Ȼ�������֤���ɣ�
�������ڽ������ʱ���ؼ���������ͻ�ƿڣ��������е�����������ʵ����ʽ����ƶϼ��ɣ�Ҫ��ͬѧ�Ǽ�ǿ�Գ����������ʵ����գ��Ա����Ӧ�ã�
��ϰ��ϵ�д�
���ϰ��
��Ŀ�����л�ѧ
��Դ��
���ͣ������
��ͥ��������һ����ѧС���磬���������ѧ֪ʶ���ش��������⣺
��1���ڳ����У����dz�����һЩӪ���Σ���ӵ��Ρ���ǿ���Ρ���ǿ���Ρ�пǿ���εȵȣ����イ�ĵ⡢�ơ�����п��ָ______��
A�����ʡ������� B�����ӡ������� C��Ԫ�ء������� D��ԭ��
��2�����ס���۵���Ҫ�ɷֶ���______��ʳ�ε���Ҫ�ɷ���______����ʹˮ�����̵�______���������״���ķ�����______��
��3��������IJ˵����������⣬�������ԭ��������______��______��Ӧ���˵�����ij��÷�����______��
��4����ϴ�ྫ������ϴȥ���ۣ�������Ϊϴ�ྫ����______���ã�
��5��ˮ���þ��˻����������һ��ˮ�����ڳ������������______����ϴ��
��6������������Ż��ˣ��ɲ�ȡ������ʩ��______��������ԭ����______��
��7������ú¯�еķ���ú��12�ס�14�ף�����16�ȶ��Ʒ�֣�����Ϊ��Խ______����ࡢ�٣�ȼ��Խ��֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
�ҹ���һ�������ڿ�������������GB/T18883-2002����2003��3��1����ʵʩ�����й涨���ڿ����м�ȩ����ѧʽΪHCHO���������ó���0.1mg/m3����ȩ�ĺ����ɸ������з�Ӧ�ⶨ��4KMnO4+5HCHO+6H2SO4�T2K2SO4+4MnSO4+5CO2+11H2O��ȡijװ������ڿ���500mL�������ʵ���������Ϊ1.58��10-8����0.00000158%���ĸ��������Һ�����м���������������Һ��300g�������еļ�ȩǡ����ȫ��Ӧ��
��1����500mL�ÿ����м�ȩ������
��2��ͨ������˵�����þ����ڿ����м�ȩ�ĺ����Ƿ���Ϲ��ҹ涨�ı���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
 ����ͼ���ø��������ȡ������װ��ͼ
����ͼ���ø��������ȡ������װ��ͼ
��1��д��ͼ�д���ŵ���������
��1��______��2��______
��3��______��4��______
��2��ָ��ͼ���Ĵ�����
��______����______��
��______����______��
��3�����ռ������жϼ���ƿ�Ѽ��������ķ���______��
��4��������ˮ���ռ���Ϻ�Ӧ��______����______��������ߵ���������______��
��5��д���÷�Ӧ�����ֱ���ʽ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
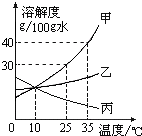 �ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ����ش�
�ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ����ش�
��25��ʱ�����ܽ��________���������������=�����ҵ��ܽ�ȣ�
��25��ʱ����25g������뵽50gˮ�У�����ܽⲢ�ָ���ԭ�¶Ⱥõ���Һ������Ϊ________g��
�����в������裺a���ܽ�b������c�����½ᾧd������Ũ�����������к��������ң����ᴿ����ȷ���������ǣ�����ĸ��ţ�________��
�ܽ�35��ʱ�ס��ҡ����������ʱ�����Һ���µ�10�棬________����ܡ����ܡ����γ���������������ȵ���Һ��
�ݽ�35��ʱ�ӽ����͵ļ���Һ��ɸ��¶��µı�����Һ���й�˵����ȷ����________
A���ܼ�������һ����С������������B�����ʵ��������ܲ���
C�����ʵ���������һ���������D����Һ������һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
��20g CaCl2��CaC03 �Ĺ���������뵽 65.9g ˮ�г���ܽ⣬������ 21.2%5Og ��Na2CO3��Һ��ǡ����ȫ��Ӧ��
��1��д���� Na2C03 ��Һ�����Ļ�ѧ��Ӧ����ʽ��
��2����ԭ���������� CaCl2 ��������
��3����������Һ��������
��4���������ɵ���Һ�������õ�t��ı�����Һ����ʱ���ܽ��Ϊ 36g/100gˮ���������������ٿ�ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ���ѡ��
�����й�ʵ��������������ȷ����
- A.
ͭ�ڿ����м��ȣ�������ɫ���棬���ɺ�ɫ����
- B.
��˿�ڿ����о���ȼ�գ������Ľ����ų����������ɺ�ɫ����
- C.
��Ũ����ƿ�����ɿ�����������
- D.
����ϵμӵ�ƺ������ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
 ijƷ����ʿ����������ǩ�IJ���������ͼ������ݱ�ǩ�ṩ����Ϣ���ش��������⣺
ijƷ����ʿ����������ǩ�IJ���������ͼ������ݱ�ǩ�ṩ����Ϣ���ش��������⣺
��1�������ɷ��У����ںϳ���ά����______��
��2���������м�����ڵ������ǣ��δ�һ����______��
��3�������Ϻ������и����һ��ͷ����ȼ���ɹ۲쵽�����������ϣ�______�����ϣ�______��
��4������÷�װ���ϵIJֿ�ʧ�𣬸���������ȼ�������е�______�����ʱһ���ѡ��______��������ţ���ͬ����������е�ʯ��Һ����ʧ��һ��ѡ��______��������ĭ������ڸɷ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��
���ͣ������
�Ȼ��ƺ�̼���ƵĻ����50����һ�������Ȼ�����Һǡ����ȫ��Ӧ������20�˳�����������Һ�����ʵ���������Ϊ20%����
��1��ԭ�������̼���Ƶ�����������
��2���Ȼ�����Һ�����ʵ�����������
�鿴�𰸺ͽ���>>

 A��B��C��D��Ϊ���л�ѧ�еij������ʣ�������֮��Ĺ�ϵ��ͼ��ʾ��ͼ�С�-����ʾ������������һ�������¿��Է�Ӧ����������ʾ���ʴ���ת����ϵ����A��B��C��Ϊ�����A��B������ͬԪ�أ�B��������ܼ����ش��������⣺
A��B��C��D��Ϊ���л�ѧ�еij������ʣ�������֮��Ĺ�ϵ��ͼ��ʾ��ͼ�С�-����ʾ������������һ�������¿��Է�Ӧ����������ʾ���ʴ���ת����ϵ����A��B��C��Ϊ�����A��B������ͬԪ�أ�B��������ܼ����ش��������⣺

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
 ����ͼ���ø��������ȡ������װ��ͼ
����ͼ���ø��������ȡ������װ��ͼ �ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ����ش�
�ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ����ش� ijƷ����ʿ����������ǩ�IJ���������ͼ������ݱ�ǩ�ṩ����Ϣ���ش��������⣺
ijƷ����ʿ����������ǩ�IJ���������ͼ������ݱ�ǩ�ṩ����Ϣ���ش��������⣺