
���������˿ڵ��������������ʳ������������������˵����ׯ��һ֦����ȫ���ʵ��ң����������ѧ��֪ʶ���ش��������⣺
��1��ũ�������������У���������������Ӫ��Ԫ������ ������Ԫ�����ƣ�
��2��ijũ��������һ�ָ��Ϸ�50kg���ø��Ϸ��к�NH4Cl��NH4H2PO4��K2SO4��������Ϊ1��2��2������ҪNH4H2PO4������Ϊ�� ��kg���ø��Ϸ��е�Ԫ�ص���������Ϊ�� ����
��3����д������NH4Cl��K2SO4�����ֻ�ѧ���ϵIJ���������ʵ�������� ��
�������� ��
�������� ��
��1�������ס��أ���2��20��10.1%��3������һ���ֱ�ȡ�������ֻ��ʣ�������Ƭ�����գ���ð�̵���NH4Cl�����������K2SO4�����������ֱ�ȡ�������ֻ�������֧�Թ��У�Ȼ���ټ�������ˮ�������Һ�����ֱ�μ�BaCl2��Һ���а�ɫ������������K2SO4�����������NH4Cl�����������ֱ�ȡ�������ֻ�������֧�Թ��У�Ȼ���ټ�����ʯ�ң����ȣ��д̼�����ζ����ų�����NH4Cl�����������K2SO4��
��������
�����������1��ֲ��������������Ҫ��Ӫ��Ԫ�رȽ϶࣬������Ҫ����������Ԫ���ǵ����ס��أ���2����Ҫ�Ȼ�淋�����Ϊ50kg�� =10kg�Ȼ���е�Ԫ�ص�����Ϊ10kg��
=10kg�Ȼ���е�Ԫ�ص�����Ϊ10kg�� ��2.62kg
�������淋�����Ϊ50kg��
��2.62kg
�������淋�����Ϊ50kg��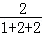 =20kg���������е�Ԫ�ص�����Ϊ20kg��
=20kg���������е�Ԫ�ص�����Ϊ20kg��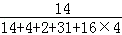 ��100%��2.43kg���Ϸ��е�Ԫ�ص���������
��100%��2.43kg���Ϸ��е�Ԫ�ص��������� ��100%=10.1%
��100%=10.1%
��3�������Ȼ�狀�����صķ����Ƚ϶࣬�磺����һ���ֱ�ȡ�������ֻ��ʣ�������Ƭ�����գ���ð�̵���NH4Cl�����������K2SO4�����������ֱ�ȡ�������ֻ�������֧�Թ��У�Ȼ���ټ�������ˮ�������Һ�����ֱ�μ�BaCl2��Һ���а�ɫ������������K2SO4�����������NH4Cl�����������ֱ�ȡ�������ֻ�������֧�Թ��У�Ȼ���ټ�����ʯ�ң����ȣ��д̼�����ζ����ų�����NH4Cl�����������K2SO4��
���㣺�������ʵ���������ã����ʵļ�����Ԫ�ص������������㣻������ɵ��ۺϼ��㣮


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com