��ҵ�����г�����NaCl��Na
2SO
4�����ʣ�������ͼװ�òⶨ��ҵ��������Ч�ɷֵĺ�����
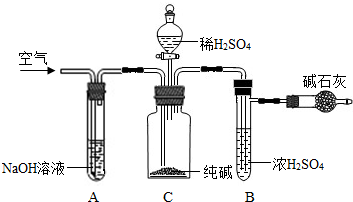
ʵ����̵���Ҫ�����ǣ�
��ȷ��ȡ��������x g��x��2����������ƿC�У���ȷ����װ�м�ʯ�ң�������CO
2���ĸ���ܵ�����y g���۴ӷ�Һ©���л���ע��ϡH
2SO
4�������ٲ�������Ϊֹ���ܻ���������������ӣ�Ȼ�����ж�£�ȷ����������z g��
���������ʵ�飬�ش��������⣺
��1��װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ
��
��2��װ��A��������
���������װ��A���ᵼ��ʵ����ƫ
�������С�����䡱����ͬ����
��3��װ��B��������
���������װ��B���ᵼ��ʵ����ƫ
��
��4���ڢܲ��л���ͨ�������������
�������ͨ��������ᵼ��ʵ����ƫ
��
��5��������Na
2CO
3�����������ļ���ʽΪ
��
��6������26.5gNa
2CO
3��NaCl�Ļ��������м���������������Ϊ10%������109.5g����ַ�Ӧ���ټ����ܶ�Ϊ1g/cm
3��������������Ϊ10%������������Һ40mL����ʱǡ����ȫ��Ӧ��������Һ��pHΪ7������
��ԭ�������̼���Ƶ�����������
��������Һ�����ʵ�����������




 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�