
£Ø1£©²ÄĮĻŹĒČĖĄąĄµŅŌÉś“ęŗĶ·¢Õ¹µÄÖŲŅŖĪļÖŹ»ł“””£
¢Ł×”Õ¬½ØÉčŠč“óĮæµÄ½ØÖž²ÄĮĻ£¬ĻĀĮŠĪļÖŹŹōÓŚø“ŗĻ²ÄĮĻµÄŹĒ”” £ØĢī×ÖÄø£©”£
a£®Ė®Äą b£®PVCĻĀĖ®¹ÜµĄ c£®²£Į§ d£®øÖ½ī»ģÄżĶĮ
¢ŚĻÖ“śÉś»īĄė²»æŖ½šŹō²ÄĮĻ£¬ĻĀĮŠøÖĢśµÄ·ĄøÆ·½·ØÖŠ£¬·ĄøÆŠ§¹ū×īŗĆ£¬µ«Ļą¶Ō·ŃÓĆŅ²×ī¹óµÄŹĒ”” ””£ØĢī×ÖÄø£©”£
a£®ĶæÓĶĘį b£®°üĖÜĮĻ²ć£Ø¶ĘĖÜ£© c£®ÖĘ³É²»ŠāøÖ
¢Ū·ĻĘ·ŹÕ¹ŗČĖŌ±·¢ĻÖ½šŹōĀĮ×öµÄŅץ¹Ž”°²»ÉśŠā”±£¬¶ųĢśÉśŠā×īĄ÷ŗ¦£¬±ćČĻĪŖ½šŹōĢś±ČĀĮŠŌÖŹ»īĘĆ£®ÄćČĻĪŖŅץ¹Ž”°²»ÉśŠā”±ŌŅņŹĒ”” ””£»ÓĆŃĪĖįæɳżČ„Ģś³ßÉĻµÄĢśŠā£ØÖ÷ŅŖ³É·ÖĪŖFe2O3£©£¬ĒėÄśŠ“³ö³żČ„ĢśŠāµÄ»Æѧ·½³ĢŹ½”” ””£»
¢Ü·Ļ¾Éµē³ŲÖŠŗ¬ÓŠ¹Æ£¬Čē¹ūĖęŅā¶ŖĘś£¬»įŌģ³ÉĪŪČ¾£¬ĶžŠ²ČĖĄą½”æµ£®¹ÆµÄŌŖĖŲ·ūŗÅŹĒ”” ””£¬½šŹō¹ÆŌŚ³£ĪĀĻĀµÄדĢ¬ŹĒ”” ”””£³£ÓƵÄøɵē³ŲÄŚ²æĢīÓŠĀČ»Æļ§ŗĶµČĪļÖŹ£¬ŌŚŹµŃéŹŅ·ÖĄėĀČ»Æļ§ŗĶ¶žŃõ»ÆĆĢ»ģŗĻĪļ£¬æɽųŠŠµÄ²Ł×÷ŹĒ£ŗČܽā”¢¹żĀĖŗĶ”” ””£»µĆµ½µÄĀČ»Æļ§ŌŚÅ©ŅµÉĻÓÖæÉÓĆ×÷”” ”””£
£Ø2£©2012Äź3ŌĀ£¬ŠĀ”¶»·¾³æÕĘųÖŹĮæ±ź×¼”·µÄ°ä²¼±ķĆ÷¹ś¼Ņ¶Ō»·¾³ĪŹĢāµÄ½ųŅ»²½ÖŲŹÓ”£
¢ŁĘū³µÉĻ¼Ó×°Ī²Ęų“߻ƾ»»Æ×°ÖĆæÉŅŌŹ¹NO”¢COĻą»„·“Ó¦×Ŗ»ÆĪŖæÕĘųÖŠŗ¬ÓŠµÄĮ½ÖÖĘųĢ壬Ćū³Ę·Ö±šĪŖ
”” ””ŗĶ”” ”””£
¢ŚĆŗČ¼ÉÕ²śÉśµÄSO2ĖłŠĪ³ÉµÄĖįÓźÖŠ£¬SO2×īÖÕ×Ŗ»Æ³ÉµÄĖįŹĒ”” £ØĢī»ÆѧŹ½£©”£ŌŚĆŗÖŠ¼ÓČėŹŹĮæŹÆ»ŅŹÆ£¬ŌŚŃõĘųµÄ¹²Ķ¬×÷ÓĆĻĀ£¬æÉŅŌÓėĆŗČ¼ÉÕ²śÉśµÄSO2·“Ӧɜ³ÉĮņĖįøĘŗĶ¶žŃõ»ÆĢ¼ĘųĢ壬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”” ”””£
£Ø3£©Ä³ÖÖŹ³Ę·µÄÅäĮĻ±źĒ©ČēĶ¼ĖłŹ¾”£
| ÅäĮĻ£ŗ Š”Āó·Ū µķ·Ū ¼¦µ° ×ŲéµÓĶ Ģ¼ĖįĒāÄĘ ±½¼ŲĖįÄĘµČ |
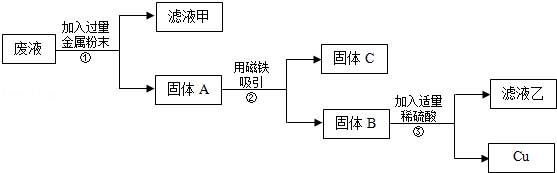
£Ø1£©¢Łd ¢Śb ¢ŪĀĮµÄ±ķĆęŅ׊Ī³ÉŅ»²ćÖĀĆܶų¼įÓ²µÄ±£»¤Ä¤£¬±£»¤ÄŚ²æµÄĀĮ²»½ųŅ»²½±»Ńõ»Æ£»Fe2O3+6HCl£½2FeCl3+3H2O ¢ÜHg£»ŅŗĢ¬£»Õō·¢£»µŖ·Ź £Ø2£©¢ŁµŖĘų£»¶žŃõ»ÆĢ¼ ¢ŚAl(OH)3
¢ŪH2SO4£»2CaCO3+O2+2SO2£½2CaSO4+2CO2£» £Ø3£©¢Ł¼¦µ°£»×Ų ÓĶ ¢Ś±½¼×ĖįÄĘ
¢ŪČ”ŹŹĮæµÄѳʷ£¬µĪ¼ÓµāŅŗ£¬Čō±ä³ÉĄ¶É«£¬ŌņŹĒµķ·Ū
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¢Ł²ÄĮĻŅ»°ć·ÖĪŖ£ŗ½šŹō²ÄĮĻ”¢ĪŽ»ś·Ē½šŹō²ÄĮĻ”¢ÓŠ»śøß·Ö×Ó²ÄĮĻ”¢ø“ŗĻ²ÄĮĻµČ£¬ŗ¬ÓŠĮ½ÖÖ»ņĮ½ÖÖŅŌÉĻ²»Ķ¬ŠŌÖŹµÄ²ÄĮĻ³ĘĪŖø“ŗĻ²ÄĮĻ”£øÖ½ī»ģÄżĶĮŹĒÓÉøÖ½īŗĶĖ®Äą»ģŗĻ³ÉµÄ£¬Ē°ÕߏĒ½šŹō²ÄĮĻ£¬ŗóÕߏĒĪŽ»ś·Ē½šŹō²ÄĮĻ£¬¹ŹøÖ½ī»ģÄżĶĮŹĒĮ½ÖÖ²ÄĮĻø“ŗĻ³ÉµÄ£¬¹ŹdÕżČ·£»
¢ŚøÖĢś·ĄøƵķ½·ØÓŠ£ŗÅēÓĶĘį”¢ĶæÓĶÖ¬”¢Åē¶Ę”¢±ķĆę¶Ū»Æ»ņ°üĖÜĮĻ²ćµČ£¬ÓĶĘįŅ×°žĀ䣬ÓĶÖ¬Ņ×»Ó·¢£¬µ«°üĖÜĮĻ²ćŠ§¹ū½ĻŗĆ£¬µ«¼Ūøń½Ļ°ŗ¹ó£¬¹ŹŃ”b£»
¢Ū½šŹōĀĮ×öµÄŅץ¹Ž”°²»ÉśŠā”±µÄŌŅņŹĒĀĮµÄ±ķĆęŅ׊Ī³ÉŅ»²ćÖĀĆܶų¼įÓ²µÄ±£»¤Ä¤£¬±£»¤ÄŚ²æµÄĀĮ²»½ųŅ»²½±»Ńõ»Æ£»ŃĪĖįŗĶŃõ»ÆĢś·“Ӧɜ³ÉĀČ»ÆĢśŗĶĖ®£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗFe2O3+6HCl=2FeCl3+3H2O£»¹Ź“š°øĪŖ£ŗĀĮµÄ±ķĆęŅ׊Ī³ÉŅ»²ćÖĀĆܶų¼įÓ²µÄ±£»¤Ä¤£¬±£»¤ÄŚ²æµÄĀĮ²»½ųŅ»²½±»Ńõ»Æ£»Fe2O3+6HCl£½2FeCl3+3H2O£»
¢Ü¹ÆµÄŌŖĖŲ·ūŗÅŹĒ£ŗHg£»½šŹō¹ÆŌŚ³£ĪĀĻĀµÄדĢ¬ŹĒ£ŗŅŗĢ¬£»ŅņĪŖĀČ»Æļ§ŗĶ¶žŃõ»ÆĆĢÖŠ¶žŃõ»ÆĆĢ²»ČÜÓŚĖ®£¬¹ŹæÉŅŌĶعż¹żĀĖ·Ø½ųŠŠ·ÖĄė£¬²Ł×÷²½ÖčĪŖ£ŗČܽā”¢¹żĀĖ”¢Õō·¢£»µĆµ½µÄĀČ»Æļ§Ņņŗ¬ÓŠÓŖŃųŌŖĖŲÖŠµÄµŖŌŖĖŲ£¬Ņņ“ĖŌŚÅ©ŅµÉĻÓÖæÉÓĆ×÷µŖ·Ź£»¹Ź“š°øĪŖ£ŗHg£»ŅŗĢ¬£»Õō·¢£»µŖ·Ź£»
£Ø2£©¢Łøł¾Ż·“Ó¦Ē°ŗóŌŖĖŲŹŲŗćÖŖ£¬NO”¢COĻą»„·“Ó¦×Ŗ»ÆĪŖæÕĘųÖŠŗ¬ÓŠµÄĮ½ÖÖĘųĢ壬ӦøĆŹĒN2ŗĶCO2£¬¹Ź“š°øĪŖ£ŗµŖĘų£»¶žŃõ»ÆĢ¼£»
¢ŚĀĮĄė×ÓŹĒČõŃōĄė×Ó£¬Ė®½āŹ±ŗĶĒāŃõøłĄė×Ó½įŗĻÉś³ÉAl(OH)3½ŗĢ壬¹Ź“š°øĪŖ£ŗAl(OH)3£»
¢Ū¶žŃõ»ÆĮņŗĶĖ®·“Ӧɜ³ÉŃĒĮņĖį£¬ŃĒĮņĖį²»ĪČ¶Ø£¬Ņ×±»æÕĘųÖŠŃõĘųŃõ»ÆÉś³ÉH2SO4£»øł¾ŻŠÅĻ¢ŹÆ»ŅŹÆŌŚŃõĘųµÄ¹²Ķ¬×÷ÓĆĻĀ£¬æÉŅŌÓėĆŗČ¼ÉÕ²śÉśµÄSO2·“Ӧɜ³ÉĮņĖįøĘŗĶ¶žŃõ»ÆĢ¼ĘųĢ壬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2CaCO3+O2+2SO2£½2CaSO4+2CO2£»¹Ź“š°øĪŖ£ŗH2SO4£»2CaCO3+O2+2SO2£½2CaSO4+2CO2£»
£Ø3£©¢Łøł¾ŻĪļÖŹĢį¹©µÄÓŖŃų³É·Ö·ÖĪö”£Š”Āó·ŪŗĶµķ·Ūø»ŗ¬ĢĒĄą£»¼¦µ°ÖŠŗ¬ÓŠµ°°×ÖŹ£»ÓĶŗĶÖ¬·¾¶¼ŹōÓŚÓĶÖ¬£¬ĖłŅŌ×Ų ÓĶ ŹĒŹōÓŚÓĶÖ¬£¬¹Ź“š°øĪŖ£ŗ¼¦µ°£»×Ų ÓĶ£»
¢Ś³£ÓĆ·ĄøƼĮÓŠ±½¼×ĖįÄĘ”¢ĻõĖįŃĪ”¢ŃĒĻõĖįŃĪŗĶ¶žŃõ»ÆĮņ£¬ĖłŅŌ±½¼×ĖįÄĘŹĒ·ĄøƼĮ£»¹Ź“š°øĪŖ£ŗ±½¼×ĖįÄĘ£»
¢ŪŅņĪŖµķ·ŪÓöµā±äĄ¶É«£¬ĖłŅŌ¼ģŃéµķ·ŪµÄ·½·ØŹĒ£ŗČ”ŹŹĮæµÄѳʷ£¬µĪ¼ÓµāŅŗ£¬Čō±ä³ÉĄ¶É«£¬ŌņŹĒµķ·Ū£¬¹Ź“š°øĪŖ£ŗČ”ŹŹĮæµÄѳʷ£¬µĪ¼ÓµāŅŗ£¬Čō±ä³ÉĄ¶É«£¬ŌņŹĒµķ·Ū”£
æ¼µć£ŗæ¼²éø“ŗĻ²ÄĮĻ”¢ÄÉĆײÄĮĻ£»»ģŗĻĪļµÄ·ÖĄė·½·Ø£»½šŹōµÄĪļĄķŠŌÖŹ¼°ÓĆĶ¾£»³£¼ūµÄ½šŹōŗĶ·Ē½šŹōµÄĒų·Ö£»ĖįÓźµÄ²śÉś”¢Ī£ŗ¦¼°·ĄÖĪ£»ÖŹĮæŹŲŗć¶ØĀɼ°ĘäÓ¦ÓĆ£»ÉśĆü»ī¶ÆÓėĮł“óÓŖŃųĖŲ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĖłŹ¾£¬µÆ»É³ÓĻĀ¹Ņ×ÅŅ»ÖŲĪļA£¬ÉÕ±ÖŠŹ¢ÓŠČÜŅŗB£¬ŹŌøł¾ŻŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ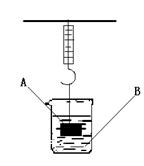
£Ø1£©ČōAĪŖĢśæ飬BĪŖĻ”ĮņĖį£¬Ōņ½«A·ÅČėBÖŠ£¬¹żŅ»»į£¬µÆ»É³ÓµÄ¶ĮŹż½«£ØĢī”°±ä“ó”±”¢”°±äŠ””±»ņ”°²»±ä”±£¬ĻĀŠ”ĢāĶ¬£© £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»
£Ø2£©ČōAĪŖĢśæ飬BĪŖĮņĖįĶČÜŅŗ£¬Ōņ½«A·ÅČėBÖŠ£¬¹żŅ»»į£¬µÆ»É³ÓµÄ¶ĮŹż½« £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ£ŠĖȤŠ”×éĶ¬Ń§ĪŖĢ½¾æĢśµÄ»ÆѧŠŌÖŹ£¬ČēĶ¼½ųŠŠĮĖŅŌĻĀČżøöŹµŃ飬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ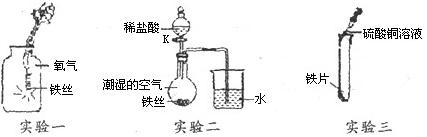
£Ø1£©ŹµŃéŅ»£ŗĖł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ £Ø2·Ö£©£¬
ŹµŃé½įŹųŗóæÉÄܲśÉśµÄ²»Į¼ŗó¹ūŹĒ £»
£Ø2£©ŹµŃ鶞£ŗ¹Ų±ÕKŅ»¶ĪŹ±¼äŗ󣬹Ū²ģµ½µ¼¹ÜÄŚŅŗĆęÉĻÉż£»“ņæŖK£¬µĪ¼ÓĻ”ŃĪĖį£¬¹Ū²ģµ½µ¼¹ÜÄŚŅŗĆęĻĀ½µ£¬µ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£¬¹Ų±ÕK£¬µ¼¹ÜÄŚŅŗĆęÉĻÉżµÄŌŅņŹĒ £»µ¼¹ÜÄŚŅŗĆęĻĀ½µµÄŌŅņŹĒ ”££ØÓĆ»Æѧ·½³ĢŹ½½āŹĶ£©£Ø2·Ö£©
£Ø3£©ŹµŃéČż£ŗŹµŃé¹Ū²ģµ½µÄĻÖĻóŹĒ£ŗ £¬
ÕāŅ»ĻÖĻóĖµĆ÷ £ØĢī”°Ģś”±»ņ”°Ķ”±£©µÄ»ī¶ÆŠŌøüĒ攣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ”×éĄūÓĆĻĀĶ¼×°ÖĆĶź³ÉO2”¢ CO»ņCO2µÄÓŠ¹ŲŹµŃé, ŹµŃé¹ż³ĢÖŠ£¬ĖūĆĒ“Óa“¦ĶØČėŅ»ÖÖĘųĢ壬ŌŚb“¦·ÅČėŅ»ÖÖ¹ĢĢ唣C“¦·ÅČėŅ»ÖÖČÜŅŗ”£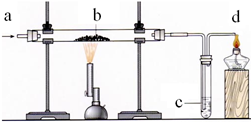
£Ø1£©Čō“Óa“¦ĶØČėO2£¬¹Ū²ģµ½b “¦·¢³ö°×¹ā£¬c“¦µÄĪŽÉ«ČÜŅŗ±ä»ė×Ē£¬Ōņb“¦·ÅÖƵÄŗŚÉ«¹ĢĢåĪŖ £¬c“¦·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©Čōb“¦¹ĢĢåÓÉŗģÉ«±äĪŖŗŚÉ«£¬ĒŅ·“Ó¦ŗóµÄ¹ĢĢåæɱ»“ÅĢśĪüŅż£¬c“¦×ĻÉ«ČÜŅŗ±äŗģ”£Ōņb“¦·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬d“¦¾Ę¾«µĘµÄ×÷ÓĆŹĒ ”£
£Ø3£©Čōb“¦¹ĢĢåÓÉŗģÉ«±äĪŖŗŚÉ«£¬b“¦·“Ó¦ŗóµÄ¹ĢĢå²»Äܱ»“ÅĢśĪüŅż£¬c“¦³ĪĒåŹÆ»ŅĖ®²»±ä»ė×Ē”£Ōņb“¦·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½šŹōŌŚÉś²śŗĶÉś»īÖŠÓŠ¹ć·ŗµÄÓ¦ÓĆ£®
£Ø1£©ČēĶ¼1½šŹōÖĘĘ·µÄÓĆĶ¾ÖŠ£¬ĄūÓĆ½šŹōµ¼ČČŠŌµÄŹĒ””””£ØĢī×ÖÄøŠņŗÅ£©£®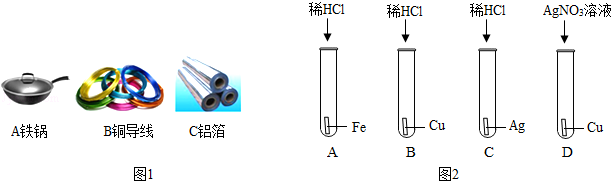
£Ø2£©ŌēŌŚĪ÷ŗŗŹ±ĘŚ£¬ČĖĆĒÖ÷ŅŖ²ÉÓĆ”°ŹŖ·ØŅ±½š”±£®Čē½«Ģś½žČėĮņĖįĶČÜŅŗÖŠ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ””
”” £¬øĆ·“Ó¦µÄ»ł±¾·“Ó¦ĄąŠĶĪŖ”” ””£»ĻÖ“ś¹¤ŅµÉĻÓĆŅ»Ńõ»ÆĢ¼ŗĶ³ąĢśæóĮ¶Ģś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”” ””£®
£Ø3£©ĪŖŃéÖ¤Fe”¢Cu”¢AgČżÖÖ½šŹōµÄ»ī¶ÆŠŌĖ³Šņ£¬Ä³»ÆѧŠĖȤŠ”×éÉč¼ĘĮĖČēĶ¼2ĖłŹ¾µÄĖÄøöŹµŃ飮ĘäÖŠ²»±Ų½ųŠŠµÄŹĒ”” ””£®
£Ø4£©Ä³µē¶Ę³§ÅŷŵÄĪŪĖ®ÖŠŗ¬ÓŠĮņĖįĶ”¢ĮņĖįŠæŗĶĮņĖįŃĒĢś£¬Ä³»ÆѧŠ”×齫Ņ»¶ØĮæµÄŠæ¼ÓČėµ½Ņ»¶ØĮæµÄøĆĪŪĖ®ÖŠ£¬³ä·Ö·“Ó¦ŗó¹żĀĖ£¬ĻņĀĖŌüÖŠ¼ÓČėŃĪĖį£¬ÓŠĘųÅŻ²śÉś£®ĀĖŌüŗĶĀĖŅŗÖŠŅ»¶Øŗ¬ÓŠµÄĪļÖŹŹĒ”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČĖĄąµÄÉś²ś”¢Éś»īĄė²»æŖ½šŹō”£
£Ø1£©ÄæĒ°Äź²śĮæ×īøߵĽšŹōŹĒ £»
£Ø2£©ĢśŌŚ³±ŹŖµÄæÕĘųĄļ»į·¢ÉśŠāŹ“£¬Ö¤Ć÷ŃõĘųŅ»¶Ø²Ī¼ÓĮĖ·“Ó¦±ŲŠėŅŖ×öµÄŹµŃéŹĒ £»
| A£®¢Ł¢Ś | B£®¢Ł¢Ū | C£®¢Ś¢Ū | D£®¢Ł¢Ś¢Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀĮ”¢ĢśŌŚČÕ³£Éś»īÖŠ¶¼ÓŠ½Ļ¹ć·ŗµÄÓĆĶ¾”£Čē£ŗ
£Ø1£©ĀĮ¾ßÓŠÓÅĮ¼µÄæ¹øÆŹ“ŠŌÄÜŹĒŅņĪŖ £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ŹŠ³”ÉĻ³öŹŪµÄ²¹ŃŖĀóʬ֊³£ŗ¬ÓŠĪ¢ĮææÅĮ£ĻøŠ”µÄ»¹ŌŠŌĢś·Ū£¬Ģś·ŪÓėČĖĢåĪøŅŗÖŠµÄŃĪĖį·¢Éś·“Ó¦×Ŗ»ÆĪŖĀČ»ÆŃĒĢś£¬Ęšµ½²¹ŃŖµÄ×÷ÓĆ£¬Š“³öÕāøö·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚŹµŃéæĪÖŠ£¬ø÷Š”×é×÷ĮĖČēĻĀŹµŃé£ŗ
| ŹµŃ鱹ŗÅ | 1 | 2 | 3 | 4 |
| ŹµŃé²Ł×÷ | 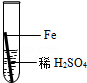 | 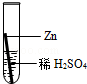 | 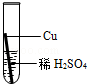 | 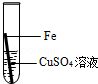 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
½įŗĻĻĀĮŠÓŠ¹ŲĢśµÄŹµŃ飬»Ų“šÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©ĢśĖæŌŚŃõĘųÖŠČ¼ÉÕµÄŹµŃé£ŗ
µćČ¼ĻµŌŚĀŻŠż×“ĻøĢśĖæµ×¶ĖµÄ»š²ń£¬“ż»š²ń½«ŅŖČ¼¾”Ź±£¬²åČėŹ¢ÓŠŃõĘųµÄ¼ÆĘųĘæÖŠ£¬¹Ū²ģĻÖĻó£®Õāøł»š²ńµÄ×÷ÓĆŹĒ”” ””£»ĪŖĮĖ·ĄÖ¹¼ÆĘųĘæµ×ÕØĮŃ£¬Ó¦²ÉČ”µÄ·½·ØŹĒ”” ””£®
£Ø2£©ČēĶ¼ŹĒĢ½¾æĢś¶¤ŠāŹ“Ģõ¼žµÄŹµŃé£ŗ
¢ŁAÖŠÖ²ĪļÓĶµÄ×÷ÓĆŹĒ”” ””£»
¢ŚŅ»¶ĪŹ±¼äŗó£¬BÖŠĢś¶¤Ć»ÓŠŠāŹ“£¬¶ųCÖŠĢś¶¤ŠāŹ“£¬ĶعżB”¢C¶Ō±ČĖµĆ÷Ģś¶¤ŠāŹ“µÄĢõ¼žÖ®Ņ»ŹĒ”” ””£®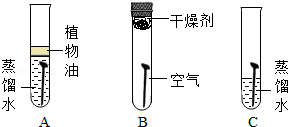
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com