
| 9.8g |
| 50g |
| X |
| 100g+X |
| 160 |
| 233 |
| y |
| 9.8g |
| 160 |
| 233 |
| y |
| 9.8g |


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?������һģ���ᡢ����Һ�ǻ�ѧ���������ʣ������������ݻش����⣺
��2013?������һģ���ᡢ����Һ�ǻ�ѧ���������ʣ������������ݻش����⣺| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 31 | 91 | 111 | 129 | 313 | 336 |
| ����NaOH��Һ�����/mL | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.0 | 7.0 | 11.0 | 12.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ����������п���ĩ��һģ�����Ի�ѧ�Ծ��������棩 ���ͣ�̽����
�ⶨ�ܽ���ж��ַ����������Dzⶨ30��ʱ����ͭ�ܽ�ȵ�ʵ�鷽�������������ϣ�30��ʱ����ͭ���ܽ��Ϊ25g/100gH2O��
һ������30��ʱ������ͭ������Һ��
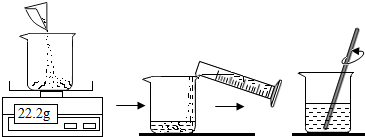
��1�����õIJ�����ͼ��

ȡ��100mLˮ��ͼ�г����õ�22.2g����ͭ����ʵ�飬�Ƿ��������˵������ ������30���ˮԡ�м�������ͭ��Һһ��ʱ�䣬��֤����ͭ��ȫ�ܽ⣬�õ�������Һ��
��������һ��������������ͭ��Һ������ͭ��������
��2��������õ�30��ʱ������Һ�У�Ѹ��ȡ��������Һ50g�����������ķ����������ͭ���������ӱ�����Һ��������ɫ����Ļ�ѧʽΪ��10�� ���õ������Ҫ�������ȣ�ֱ��������ɫ��Ϊ��11�� ɫΪֹ���˲��������У��õ�������������̨���ƾ����⣬������12�� ��
��3���������õ���������Ϊ9.8g��������õ���ʱ����ͭ���ܽ��Ϊ��13�� g/100gH2O����ȷ��0.1g����ʵ�鷴˼��ʵ����ֵ�������е�����ƫС�����ܵ�ԭ������14�� ��д��һ�����ɣ���
��4������50g��������ͭ��Һ������ͭ��������������ʹ����������
ʵ��ԭ����CuSO4+BaCl2 ��CuCl2+BaSO4��
��ʵ�鷽����һ����Ҫ�õ���ʵ���������15�� ����д�������ƣ��������յõ����ᱵ14.5g��������Һ������ͭ������Ϊ���ٿˣ�����ʾ������ͭʽ��Ϊ160�����ᱵʽ��Ϊ233��д��������̣���ȷ��0.1g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ᡢ����Һ�ǻ�ѧ���������ʣ������������ݻش����⣺
�±����ڲ�ͬ�¶����������Ƶ��ܽ�ȣ�
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 31 | 91 | 111 | 129 | 313 | 336 |
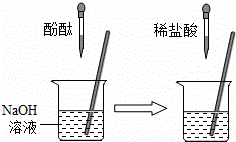
��1������������Һ�е�������������20��ʱ����100��ˮ�м���100���������ƹ��壬������ҺΪ������Һ������͡������͡���������Һ�������������ˣ�
��2����ѧϰ�����У������жϷ�Ӧ�����ķ����ж��֣�
����ʢ��10mLϡ������ձ��м�������������Һ����pH�ƣ���pH���������ⶨ��Һ��pH�������������£�
| ����NaOH��Һ�����/mL | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.0 | 7.0 | 11.0 | 12.2 |
����������������Һ�����Ϊ����mLʱ��ϡ���������������Һǡ����ȫ��Ӧ��
������ͼ��ʵ���У����۲쵽������������ �Ϳ�֤��NaOH��HCl�����˷�Ӧ��
�۲�ʹ��ָʾ����pH��ֽ�ȷ�����Ҳ��֤����Ӧ��������ϡ������������ƻ�Ϻ����Һ�м���һ�����ʣ���û������������֣�˵����Һʧȥ�����ԣ��Ӷ�֤��ϡ��������������Ѿ������˷�Ӧ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ⶨ�ܽ���ж��ַ����������Dzⶨ30��ʱ����ͭ�ܽ�ȵ�ʵ�鷽�������������ϣ�30��ʱ����ͭ���ܽ��Ϊ25g/100gH2O��
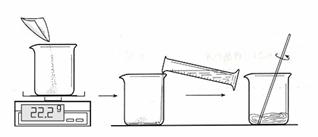 һ������30��ʱ������ͭ������Һ��
һ������30��ʱ������ͭ������Һ��
�����õIJ�����ͼ��
ȡ��100mLˮ��ͼ�г����õ�22.2g����ͭ����ʵ�飬�Ƿ��������˵��������9�� ������30���ˮԡ�м�������ͭ��Һһ��ʱ�䣬��֤����ͭ��ȫ�ܽ⣬�õ�������Һ��
��������һ��������������ͭ��Һ������ͭ��������
��������õ�30��ʱ������Һ�У�Ѹ��ȡ��������Һ50g�����������ķ����������ͭ���������ӱ�����Һ��������ɫ����Ļ�ѧʽΪ��10�� ���õ������Ҫ�������ȣ�ֱ��������ɫ��Ϊ��11�� ɫΪֹ���˲����� ���У��õ�������������̨���ƾ����⣬������12�� ��
���У��õ�������������̨���ƾ����⣬������12�� ��
���������õ���������Ϊ9.8g��������õ���ʱ����ͭ���ܽ��Ϊ��13�� g/100gH2O����ȷ��0.1g����ʵ�鷴˼��ʵ����ֵ�������е�����ƫС�����ܵ�ԭ������14��
��д��һ�����ɣ���
�ܲ���50g��������ͭ��Һ������ͭ��������������ʹ����������
ʵ��ԭ����CuSO4+BaCl2 ��CuCl2+BaSO4��
��ʵ�� ������һ����Ҫ�õ���ʵ���������15�� ����д�������ƣ��������յõ����ᱵ14.5g��������Һ������ͭ������Ϊ���ٿˣ�����ʾ������ͭʽ��Ϊ160�����ᱵʽ��Ϊ233��д��������̣���ȷ��0.1g��
������һ����Ҫ�õ���ʵ���������15�� ����д�������ƣ��������յõ����ᱵ14.5g��������Һ������ͭ������Ϊ���ٿˣ�����ʾ������ͭʽ��Ϊ160�����ᱵʽ��Ϊ233��д��������̣���ȷ��0.1g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com