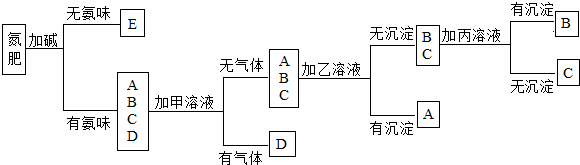
£Ø2010?ČÕÕÕ£©Ēėøł¾ŻĻĀĶ¼»Ų“šĪŹĢā£ŗ
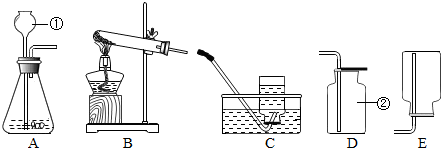
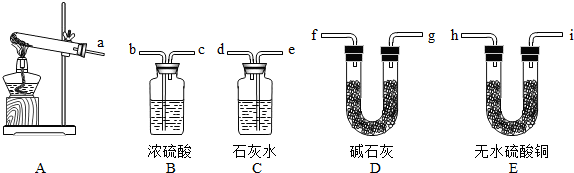
£Ø1£©Ķ¼ÖŠ±źÓŠ¢Ł¢ŚµÄŅĒĘ÷Ćū³Ę£ŗ¢Ł
³¤¾±Ā©¶·
³¤¾±Ā©¶·
£®¢Ś
¼ÆĘųĘæ
¼ÆĘųĘæ
£®
£Ø2£©ÓĆŹÆ»ŅŹÆŗĶĻ”ŃĪĖįÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½ŹĒ
CaCO3+2HCl=CaCl2+CO2ӟ+H2O
CaCO3+2HCl=CaCl2+CO2ӟ+H2O
£®æÉŃ”ÓĆĶ¼ÖŠ
A
A
ŗĶ
D
D
£ØĢīŠņŗÅ£©×é×°Ņ»Ģ×ÖĘČ”¶žŃõ»ÆĢ¼µÄ×°ÖĆ£®¼ģŃ鶞Ńõ»ÆĢ¼¼ÆĀśµÄ·½·ØŹĒ
ÓĆŅ»øłČ¼ÉÕµÄľĢõĘ½·ÅŌŚ¼ÆĘųĘææŚ£¬Ä¾ĢõĻØĆš£¬Ö¤Ć÷ĀśĮĖ
ÓĆŅ»øłČ¼ÉÕµÄľĢõĘ½·ÅŌŚ¼ÆĘųĘææŚ£¬Ä¾ĢõĻØĆš£¬Ö¤Ć÷ĀśĮĖ
£®
£Ø3£©°Ń×°ÖĆAŗĶ×°ÖĆCĻąĮ¬×é³ÉŅ»Ģ××°ÖĆ£¬øĆ×°ÖĆæÉÖĘČ”µÄŅ»ÖÖĘųĢåŹĒ
ŃõĘų»ņ£ØĒāĘų£©
ŃõĘų»ņ£ØĒāĘų£©
£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
2H
2O
22H
2O+O
2”ü£Ø»ņZn+H
2SO
4=ZnSO
4+H
2”ü£©
2H
2O
22H
2O+O
2”ü£Ø»ņZn+H
2SO
4=ZnSO
4+H
2”ü£©
£®
£Ø4£©ĻąĶ¬Ģõ¼žĻĀ£¬°±Ęų£ØNH
3£©µÄĆܶȱČæÕĘųŠ”£¬ĒŅŅ×ČÜÓŚĖ®£»¼ÓČČ£¬ĀČ»Æļ§ŗĶĒāŃõ»ÆøĘ¹ĢĢå»ģŗĻĪļæÉÖĘČ”°±Ęų£®ŌņÖĘČ”°±ĘųӦєŌńµÄ×°ÖĆŹĒ
B
B
£ØĢīŠņŗÅ£©£®