
������ͼ��ʾ,�ش��й�����:
|
��1��д��ͼ�б�����ĸ���������ƣ�a ��
��2��ʵ�����ø��������ȡ������Ӧѡ�õķ���װ���� ������ĸ����ʵ��ʱ��װ���Թܿ�Ӧ��һ��������Ŀ���� ����Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ������ȡ������̼���壬��Ҫ��ø���Ķ�����̼��������װ���⣬��Ӧѡ��Dװ�ã�װ���е�Һ���� ����д�Լ����ƣ��������Eװ���ռ������壬������Ӧ�� �˽��루�b����c������ͨ���ó���ʯ��ˮ�����������̼����Ӧ�Ļ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�챱���з�ɽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��8�֣�����������������벻��������
��1����ͭ�Ƶ�����Ҫ������ͭ�������õ� �ԡ�
��2�����dz��á�ͭǽ���ڡ�����������ļ�̡�������һ��������Ҳ�ܷ������ַ�Ӧ��
����˿��������ȼ�գ���Ӧ�Ļ�ѧ����ʽ��________________________________��
��3������Ʒ��ʴ��ʵ���������������е� �����˻�ѧ��Ӧ����ֹ����������
ʴ��һ�ַ����� ________________________ ��
��4��������ͼ��ʾ�ش�������һ���еĽ����� ____ ��������ϡ���ᷴӦ�Ļ�ѧ��
��ʽΪ_______________________________________��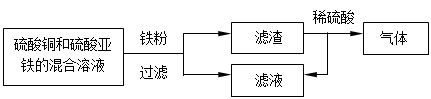
��5����ijϡ�����Ϊ����������ݣ����������ձ��У��ֱ�����������п�������ֽ�
������Ӧ�������û��ʣ��,���������������淴Ӧʱ��仯������ͼ������˵��
��ȷ����___________��������ĸ��ţ�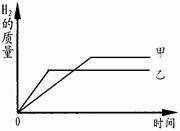
| A��������������п |
| B�������������������� |
| C���μӷ�Ӧ��п������С���������� |
| D����ַ�Ӧ��ϡ����һ������ʣ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������벻��������
��1����ͭ�Ƶ�����Ҫ������ͭ�������õ� �ԡ�
��2�����dz��á�ͭǽ���ڡ�����������ļ�̡������� һ��������Ҳ�ܷ������ַ�Ӧ��
һ��������Ҳ�ܷ������ַ�Ӧ��
����˿��������ȼ�գ���Ӧ�Ļ�ѧ����ʽ��________________________________��
��3������Ʒ��ʴ��ʵ���������������е� �����˻�ѧ��Ӧ����ֹ����������
ʴ��һ�ַ����� ________________________ ��
��4�������� ͼ��ʾ�ش�������һ���еĽ����� ____ ��������ϡ���ᷴӦ�Ļ�ѧ��
ͼ��ʾ�ش�������һ���еĽ����� ____ ��������ϡ���ᷴӦ�Ļ�ѧ��
��ʽΪ_______________________________________��
 |
��5����ijϡ�����Ϊ����������ݣ����������ձ��У��ֱ�����������п�������ֽ�
������Ӧ�������û��ʣ��,���������������淴Ӧʱ��仯������ͼ������˵��
��ȷ����___________��������ĸ��ţ�
A��������������п
B��������������������
C���μӷ�Ӧ��п������С����������
D����ַ�Ӧ��ϡ����һ������ʣ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾ,�ش��й�����:
|
��1��д��ͼ�б�����ĸ���������ƣ�a ��
��2����װ��A��E������ȡ���ռ���������װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��ʵ��ʱ��װ���Թܿ�Ӧ���� ������װ��E�ռ�����ʱ������Ӧ�� �˽��루�b����c��������װ���ռ�����ʱ��������������� ��
��3��ʵ������ȡ������̼���壬ѡ��Cװ�������ѡ��Bװ�õ��ŵ��� ��
��4����Dװ����Ϊϡ���������Һ���ڴ���Һ��ͨ��SO2����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
|
��1��д��ͼ�б�����ĸ���������ƣ�a ��
��2��ʵ�����ø��������ȡ������Ӧѡ�õķ���װ���� ������ĸ����ʵ��ʱ��װ���Թܿ�Ӧ��һ��������Ŀ���� ����Ӧ�Ļ�ѧ����ʽΪ ��ʵ�������ֹͣ����ǰҪ�Ƚ������Ƴ�ˮ�棬Ŀ������ ��
��3��ʵ������ȡ������̼����ʱ�����Eװ���ռ������壬������Ӧ�� �˽��루�b����c��������д��ʵ������CO2�Ļ�ѧ��ʽ ��ͨ���ó���ʯ��ˮ�����������̼����Ӧ�Ļ�ѧ����ʽΪ
��
�������װ��ѡ��C�����ŵ��� ��
��4����Dװ����ʢ����ɫʯ����Һ��ͨ��CO2�������Һ�� ��ԭ���� �����û�ѧ����ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
|
��1��д��ͼ�б�����ĸ���������ƣ�a ��
��2��ʵ�����ø��������ȡ������Ӧѡ�õķ���װ���� ������ĸ����ʵ��ʱ��װ���Թܿ�Ӧ��һ��������Ŀ���� ����Ӧ�Ļ�ѧ����ʽΪ ��ʵ�������ֹͣ����ǰҪ�Ƚ������Ƴ�ˮ�棬Ŀ������ ��
��3��ʵ������ȡ������̼����ʱ�����Eװ���ռ������壬������Ӧ�� �˽��루�b����c��������д��ʵ������CO2�Ļ�ѧ��ʽ ��ͨ���ó���ʯ��ˮ�����������̼����Ӧ�Ļ�ѧ����ʽΪ
��
�������װ��ѡ��C�����ŵ��� ��
��4����Dװ����ʢ����ɫʯ����Һ��ͨ��CO2�������Һ�� ��ԭ���� �����û�ѧ����ʽ��ʾ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com