
�������ͼ��ʾʵ��װ�ûش����⣺
��1��д��ͼ�б�����������ƣ�a�� ��b�� ��
��2������װ��B��ȡ������ҩƷѡ�ø�����أ�������Ӧ�Ļ�ѧ����ʽΪ �����Թܿڷ�һ������������ ��ʵ�鿪ʼǰ��ͼF��ʾ��������־��װ�����������õ������� ��
��3��ʵ��������װ��A��ȡ������̼���壬������Ӧ�Ļ�ѧ����ʽΪ ������4g CaCO3�μӷ�Ӧ�������� g CO2���壮ʵ�����ռ�������̼ѡ��װ�� ������ţ������ռ�ij����ֻ�ܲ���װ��E���ɴ��Ʋ��������е����������� ��
��1���ƾ��ƣ�����ƿ ��2��2KMnO4 K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������� ��3��CaCO3+2HCl��CaCl2+H2O+CO2����1.76��D��������ˮ���ܶȱȿ���С
K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������� ��3��CaCO3+2HCl��CaCl2+H2O+CO2����1.76��D��������ˮ���ܶȱȿ���С
���������������ͼ����֪�����������ƣ�������طֽ�����������ء��������̺�������������ֹ�������С�������뵼�ܣ�װ�����������ã��������Թ��¶����ߣ����������ݳ���������ݣ�ʵ������ȡ������̼ʹ�õ���̼��ƺ����ᷴӦ�����ݷ�Ӧ�Ļ�ѧ����ʽ����������ɶ�����̼�������������ռ�װ�õ�ѡ��ȡ����������ܶȺ��ܽ��ԣ��ݴ˽�ɡ�
��1����ͼ��֪��a�Ǿƾ��ƣ�b�Ǽ���ƿ������ƾ��ƣ�����ƿ��
��2��������������ֽܷ���������ء��������̺��������ڴ�װ���У��Թܿ�Ҫ��һ��������ֹ�������С���������뵼�ܣ��������Թܣ����Թ����¶����ߣ����������ݳ���װ�����������ã����������������2KMnO4 K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������ݣ�
K2MnO4+MnO2+O2��������KMnO4���뵼�ܣ����ܿ������ݣ�
��3��ʵ������ȡ������̼ʹ�õ���̼��ƺ����ᣬ�����ܷ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�������ɶ�����̼������Ϊx
CaCO3+2HCl��CaCl2+H2O+CO2��
100 44
4g x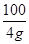 ��
��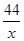
���x��1.76g
������̼������ˮ������ʹ����ˮ���ռ���������̼���ܶȱȿ�����ʹ�������ſ������ռ���ֻ��ʹ��Eװ���ռ������壬��������ˮ���ܶȱȿ���С�����壬���CaCO3+2HCl��CaCl2+H2O+CO2����1.76��D��������ˮ���ܶȱȿ���С��
���㣺���鳣������ķ���װ�ú��ռ�װ����ѡȡ����


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������װ�ã��ش��������⣺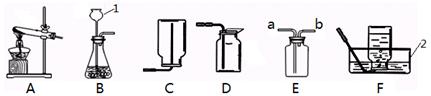
����д��ͼ�б�����ĸ���������ƣ�1�� 2��
��ʵ�����ø��������ȡ�������ռ�������������Ӧѡ�õ�װ������� ������ĸ����
��д���÷�Ӧ�ı���ʽ�� ��
ʵ��ʱ��װ���Թܿ�Ӧ��һ��������Ŀ���� ��
�ø�װ���ռ���������ʱ���� �� ����ѡ��ٽ������Ƴ�ˮ�� ��Ϩ��ƾ��ƣ�
��������Ŀ���� ��
��ʵ�����ù���������Һ��ȡ���������õķ���װ���� ����д����Ӧ�ı���
ʽ�� ������Dװ���ռ���������д��������
������ ��
�ܲ������ϵ�֪������(NH3)��һ���ܶȱȿ���С�Ҽ�������ˮ�����塣����ѡ�ü����Ȼ�狀��������ƵĹ�����������ȡ����ʱ����ѡ��ķ���װ���� ��
�������Eװ���ռ������������ӵ��� ���a����b������ͨ�롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ��Ӧ�����Ŀ�����ʵ����ꡱ��ijУ��ѧ��ȤС������ʦָ���£�̽���ˡ�Ӱ���������ֽ��ٶȵ����ء�������ش��������⡣
��1��ȡ��֧���Թܣ��ֱ���������5%��10%�Ĺ���������Һ���ټ�������Ķ������̣����ռ�һС�Թ����壬����Ũ�ȴ�����ռ������ò��������к���������˳�� ����ѡ����ţ�
�ټ���װ�õ������� ���������е��ܵĵ�����
�۽�ˮ���д��ռ������С�Թ�ע��ˮ ���������ȶ���������ˮ����
������Թ��ڵ�����������������Һ�ټ��������������̷�ĩ
��2��ȡa ��b��֧�Թܼ�������5%�Ĺ���������Һ���ٷֱ���������������̷�ĩ������ͭ��CuO����ĩ������a �б�b�в������ݶ��ҿ졣�漴�ô����ǵ�ľ���ֱ����������Թ��ڣ�����a�л��Ǹ�ȼ��b�л��ǽ�����������ȼ���ɴ˵ó�Ӱ�����������Һ�ֽ�������� ��
��3��ijͬѧ����̽������ש��ĩ�Ƿ�Ҳ��������������ֽ�Ĵ�������ʵ�鲽����������£�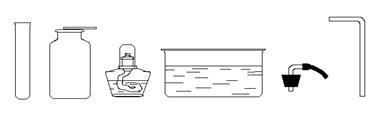
a b c d e f
�����ֱ�����֧�Թ��м����������Ũ�ȵĹ���������Һ��������һ֧�Թܼ���һҩ��ש��ĩ��Ȼ����֧�Թ��е�����ͨ��ˮ�бȽϲ������ݵĿ��������ּ����ש��ĩ���Թ��з�Ӧ�Ͽ졣
�ڽ���Ӧ�Ͽ���Թ��ڹ�����˳�����ϴ�ӡ���ɡ�
���ú�ɺ�Ĺ����ظ�����ٵ�ʵ�飬�����벽�����ȫ��ͬ��
�Իش𣺲������������ѡ���Թ���õ������г��������� ������ĸ��������۵�ʵ��Ŀ���� ����ѧ����Ϊͨ������ʵ����֤����ש��ĩ��������������ֽⷴӦ�Ĵ���������ʦ��Ϊ��ͬѧ��ʵ�黹ȱ��һ���ؼ����裬��ָ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ���������µĻ�ѧѧϰ���������Ѿ�����������ʵ������ȡһЩ�������֤�������ʵ��й�֪ʶ������ͼʾ�ش�����
��1��д��ͼ�б�ʾ���������ƣ��� ���� ��
��2��ʵ����������غͶ���������ȡ����Ӧѡ�õķ���װ��Ϊ ������ĸ��ţ���ͬ����O2����ͼ��F��Gװ���ռ�����Fװ���ռ�������Ϊ________________��������________________������ʱ��˵������������д���÷�Ӧ�Ļ�ѧ����ʽ __________ ��
��3��ʵ���ҳ��ù���������Һ��ȡ�������÷����������������ŵ㣬�磺___(дһ��)
��4��I��J����֤�������ʵIJ���ʵ�飬����ʵ���У�ʵ��ǰ����ƿ�ﶼװ��������ˮ�����У�I����ˮ�������� ��J����ˮ�������� ��I�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��5��ע����C�ڻ�ѧ�е����úܶ࣬�磺�����ڼ��װ��E�������ԡ��������£�������ƿ�м�������ˮ��û����©���¶ˡ���ע����C���ӵ�װ��E�ĵ��ܿڴ��������ƶ�ע����C�Ļ��������װ��E�����������ã��۲쵽�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ���ҳ��ÿ�״������(FeS)��ϡ�����ڳ����·�Ӧ��ȡ����(H2S)����������ʣ�����ɫ���г�������ζ���ж����壬���ܶȴ��ڿ������ڿ���ȼ�գ�ȼ��ʱ����ˮ�Ͷ����������壻��������ˮ���������������Ʒ�Ӧ��������������ݺ�����װ��ͼ�ش�����(װ������ĸ��ʾ)��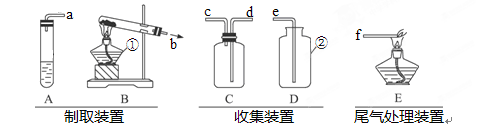
(1)д��������������ƣ���____________ ��____________
(2)��ȡH2Sѡ��_________���ռ�ʱѡ��_________���ռ�ʱH2S����Ӧ��________��(����ܴ���)���뼯��ƿ���ռ������Ժ���H2S����ļ���ƿӦ�� ������š����á�)��ʵ��̨�ϡ�
(3) H2Sȼ�յĻ�ѧ����ʽ��___________ ___������Eװ�ô���β����?Ϊʲô? ____________________________________________��
(4)AD��ϻ���������ȡ���������û�ѧ����ʽ��ʾ�÷�Ӧԭ�� ����������Dװ���ռ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ѿ�ѧϰ��ʵ������ȡһЩ����ķ�Ӧԭ��������װ�ü��ռ������������ͼ��������ĿҪ��ش����⡣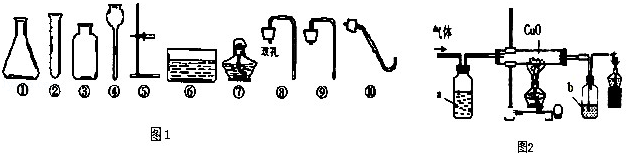
��1��д��ͼ1�����������ƣ��� ���� ��
��2������ô���ʯ��ϡ������ȡ������̼������װ�ÿ�ѡ����ͼ�����е� ������ţ����仯ѧ����ʽ ��
��3�����ü��ȹ���KMnO4����ȡ������ˮ���ռ�һƿO2������װ�ú��ռ�װ�ÿ�ѡ��ͼ1�����е� ������ţ���д�����������IJ��������� ��
��4��ijͬѧ�ú���CO2��CO����ԭCuO�������������CO2�����������ͼ2��ʾ��ʵ��װ�ã��뽫����Ƶ�װ��ͼ��������������ͼ2�У� ��ͼ���Լ�a����� ��ѡ �NaOH��Һ����Ca(OH)2��Һ����װ��ĩ�˷�һȼ�յľƾ��Ƶ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ͼ��ʾʵ��װ�ûش����⣺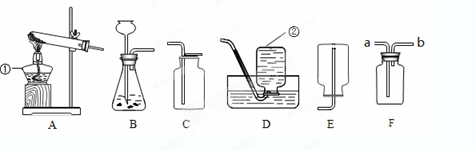
��1��ָ��ͼ�б������ֵ��������ƣ��� ���� ��
��2��ʵ�������ø��������ȡ��������ѡ�õķ���װ���� ������ţ�����Ӧ�Ļ�ѧ����ʽΪ ����Ҫ�ռ���Ϊ�������������ѡ�� װ�ã�����ţ���ʵ��ʱӦ���Թܿڷ�һ�������������� ���Թ��е���û��ȼ�գ�����ȼ�յ�������������Ҫԭ���� ��
��3��ʵ�����ô���ʯ��ϡ������ȡ������̼����װ��Ӧѡ�õ�װ��Ϊ ������ţ�����Ӧ�Ļ�ѧ����ʽΪ ������Ҫ���Ʒ�Ӧ�����ʣ���װ���Ͽ��������Ľ��� ������Cװ���ռ�������̼��ԭ���� ��
��4��Fװ����һ�ֿ����ڼ�����ϴ���ȵĶ��װ�á�����Fװ����װ��ˮ����������Ͳ���Ϳ������ڲⶨ������ˮ�Ҳ���ˮ��Ӧ�����������������Ӧ�� ���a����b��������F�С�
��5������Fװ�ü������ɵ������Ƿ��Ƕ�����̼��������Fװ���м��� ������Ӧ�� ���a����b��������F�С���Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������ȡ��������װ������ͼ��ʾ�� 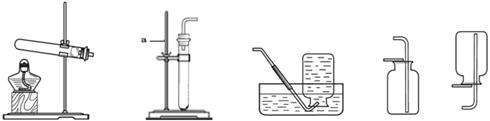
A B C D E
��ش��������⣺
��1��װ���бꡰa�������������� ��
��2�����оƾ���ʹ�÷�������ȷ���� ������ţ���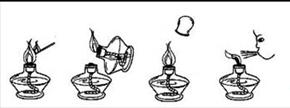
A B C D
��3���ø��������ȡ���������ֱ���ʽ�� ����ѡ�õķ���װ���� ������ţ����ռ��ϴ���������Ӧѡ����ռ�װ���� ������ţ���
��4���ù���������Һ��ȡ���������ֱ���ʽ�� ��Ӧѡ�õķ���װ���� ��
��5������Dװ���ռ�����ʱ�������Ƿ��ռ����ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��7�֣�����ͼʾΪ���������Ʊ������������ʵ��IJ���������������ĿҪ�ش��������⣺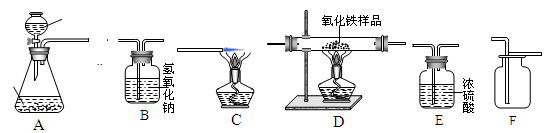
��1������ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ (��д���������ĸ)��
������A�У����������Ļ�ѧ����ʽΪ ��
��2�����ú�����CO2 ���ʵ�һ����̼���壬�ⶨij��������Ʒ�Ĵ���(���ʲ���Ӧ)����ѡ����������˳��Ϊ��D��B��C��
�ٸ�װ��D����ǰӦ����ͨһ���һ����̼���壬��������ԭ���ǣ�
��
��װ��C�������� ��
��װ��B�У�������Ӧ�Ļ�ѧ����ʽΪ ��
�ܳ�ַ�Ӧ����Dװ�ü���2.4�ˣ�����Ʒ��������������Ϊ �ˡ�
����ͨ��������Ӧǰ����Bװ�õ������仯����������Ʒ������������������������� ��(�ƫС����ƫ�� ��������Ӱ�족����֮һ)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com