
ҽ���Ȼ��Ƴ����ںϳ�ҩ��Թ�ҵ̼���(������Fe3+������)Ϊԭ��������ˮ���Ȼ���
(CaCl2��2H2O)������ͼ������ʾ��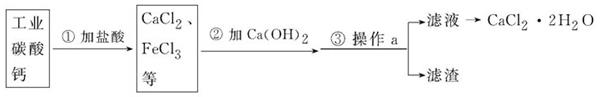
(1)д���ڢٲ���̼��������ᷴӦ�Ļ�ѧ����ʽ�� ��
(2)�ڢڲ����Ƿ�����ѧ�仯�� (��ǡ���)��
(3)����a�������� ��ʵ���ҽ��иò���ʱ�������������� ��
(4)���������������������Σ���Ҫ�����㹻�ĸƣ�д��һ�ֺ����IJ��Ʒ����� ��
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��6�֣���ͼ�dz��л�ѧ�г������ʼ��ת����ϵ������ֻ��FΪ���ʣ��������ǻ����B��C��D����������DΪ��ɫ��ͼ�С�������ʾ���˵������ܷ�����ѧ��Ӧ����������ʾ���ʼ����ת����ϵ�����෴Ӧ���������ַ�Ӧ��������������ȥ��
��1��д��D���ʵĻ�ѧʽ ��D��F��Ӧ�����Ļ�����Ӧ���� ��
��2��д��G��E�Ļ�ѧ����ʽ�� ��
��3��д��A��B+C�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��������������Ϣ���ش��������⡣��������ĸ���������ʾ�Ϊ���л�ѧ�������ʣ���
��1��A��B����������ͬԪ����ɡ�
�� ��A�����ֽⷴӦ������B����A�Ļ�ѧʽΪ ��
�� ��A�������Ϸ�Ӧ������B����÷�Ӧ�Ļ�ѧ����ʽΪ ��
�� ��D�ֱ��벻ͬ�����������ʷ�����Ӧ������A��B���䷴Ӧ��ѧ����ʽ�ֱ�Ϊ ;
��
��2��E��F��G����3��Ԫ����ɡ�E��F��E��G�ܷ�����ѧ��Ӧ��F��G���ת�������������ڲ�ͬ�����ʷ��ࣨ�ᡢ��Ρ�������������ǵĻ�ѧʽ�ֱ�ΪE ��F ��G ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
������ѧϰ����Ҫ������С���ڸ�ϰ���������ʱ�����ɳ������������ѧ���ʡ�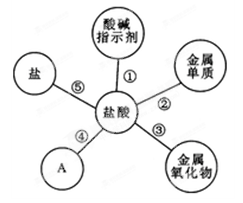
��1��Ϊ����֤���ʢ٣�С�콫��ɫʯ����Һ�μӵ�������Һ�У���Һ�� ɫ��
��2��ͼ��A����ʾ����������� ������д��һ������ʽ ��
��3����������ʢ۾��������������������⣬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4���������ʢݣ�������ʵ������ȡ������̼��д������ʽ ��
��5��þ��п����������֤��������ʢڣ�С��Ҫ̽��þ��п�����ᷴӦ�Ŀ�������Ҫ���Ʋ��䣨��ͬ������ ������ţ���
| A�����ֽ�������״ | B��������������� |
| C����Ӧ�����Ĵ�С | D���¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��һ�ֹ�ҵ��ˮ�����д�����ZnSO4������CuSO4�Լ����ࡣ��ȤС���ͬѧ����л��ս���ͭ������п���壬������������·�����
��1������a��b�������� ����Ŀ���dz�ȥ��Һ�� ��������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͬѧ���X���ý���������ͬѧ��ΪX���ý���п������Ϊ ����ס����ҡ���ͬѧ���������ȷ�ģ������� ��������м�������Ľ���X��Ŀ���� ��
��3������Һ1����Һ2������ZnSO4�����������ֱ��ʾΪa��b����a��b�Ĵ�С��ϵ��__________����Һ3�е������� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
ij������CaSO4��NH3��H2O��CO2�Ʊ���NH4��2SO4���乤���������£�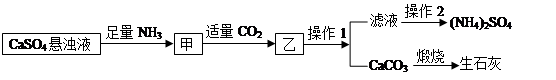
�����ƶϲ���������
| A������1������2������ |
| B���������̵��ܷ�Ӧ�ķ���ʽΪ��CaSO4 +2NH3 +CO2 +H2O=��NH4��2SO4 +CaCO3�� |
| C����������CO2�ɱ�ѭ��ʹ�� |
| D���˹���������ͨCO2��ͨNH3���������Ч�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
�о�С��Ϊ�˽ⱱ����ɽ�ĵ����������ʵ��ѡȡ������ƷD
����ƷJ������������ʾ��ʵ�飨���в��ַ�Ӧ���ﱻ��ȥ����
��1�����ƶϳ��������ʵĻ�ѧʽ
A ��C ��D ��F ��G ��J ��
��2�����ͼ�����õ�Xֻ������Ԫ�أ��볢��д������ת���Ļ�ѧ����ʽ��
D+X���� ��
D+E���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
ij��������M���ܺ���Na2CO3��NaOH��NaCl��Na2SO4�е�һ�ֻ��֣�ѧ��Ϊ̽��M��������������ʵ�飬�����ʵ�鲹�����в���ʵ�����������ͽ��ۣ���������ʵ�鱨�森
| ʵ�����ݼ��������� | ʵ������ | �� �� |
| ��1��ȡ����M��Ʒ���Թ��У�����������ˮ�ܽ⣬Ȼ����뼸�η�̪��Һ�� | ��Һ��Ϊ��ɫ | ˵��M����Һ���� ���ԣ� |
| ��2��������������Һ�е����������ᣮ | ��Һ��Ϊ��ɫ��������ų��� | ԭM�п϶������� ���� �϶������� ���� |
| ��3����ȡ����M��Ʒ���Թ��У�����������ˮ�ܽ⣬Ȼ������������� ����Һ������ϡ���ᣬ���ˣ� | �а�ɫ��������������ɫ��Һ�� | ԭM�п϶�����Na2SO4�� |
| ��4�������棨3��������ɫ��Һ�е��뼸��AgNO3��Һ�� | �� �� | ԭM�п϶�����NaCl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҡ������ֹ������ʵ��ܽ��������ͼ��ʾ���ش��������⣺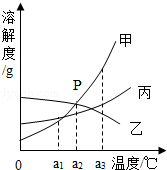
��a3��ʱ���������ʵ��ܽ�������ɴ�С��˳������ ����
��a2��ʱ��P��ĺ����� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com