

| 56 |
| x |
| 160 |
| 16g |
| 160 |
| 16g |
| 64 |
| y |
| 160 |
| 16g |
| 152 |
| z |
| 5.6g |
| 6g |
| 15.2g |
| 5.6g+160g-6.4g+m |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���Ͽ����Ƚ���ֹⷳ�ʣ���ͼ�г������ϵ�Ӫ���ɷ֣�
���Ͽ����Ƚ���ֹⷳ�ʣ���ͼ�г������ϵ�Ӫ���ɷ֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| һ���¶ȡ�ѹǿ |
| A��O2 |
| B��CO |
| C��C2H2 |
| D��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ���������� | ���� |
| A | O2��CO2 | �ֱ�ͨ�����ʯ��ˮ�� |
| B | HCl��H2SO4 | �ֱ�μ�BaCl2��Һ |
| C | Ca��OH��2��Һ��Na2CO3��Һ | �ֱ�μӷ�̪��Һ |
| D | CuCl2��Һ��Cu��NO3��2��Һ | �ֱ�μ�AgNO3��Һ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
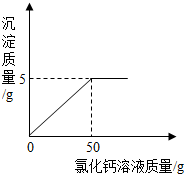 ��ҵ����̼������Һ��ʯ��ˮ��Ӧ���ռ����̼������Һ��ʯ��ˮ�Ƿ�ǡ����ȫ��Ӧ��ij��ѧ��ȤС���ͬѧ���������й��ˣ�������Һ��������̽����
��ҵ����̼������Һ��ʯ��ˮ��Ӧ���ռ����̼������Һ��ʯ��ˮ�Ƿ�ǡ����ȫ��Ӧ��ij��ѧ��ȤС���ͬѧ���������й��ˣ�������Һ��������̽����| ʵ�鲽�� | ʵ������ | ���� |
| ȡ������Һ�����Թ��У��ٵ����Ȼ�����Һ | �����ɰ�ɫ���� | ̼���ƹ��� |
| ������������ | ǡ�÷�Ӧ |
| ʵ�鲽�� | ʵ������ͽ��� |
| 1 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2014��4��2�ա�4�շ������廪ѧ��PX����������ս���������ڰٶȰٿ���������PX��Ϊ���Ͷ��������ȷ����������˽�ʾ��������ʵ�Ŀ�ѧ̬�ȣ�PX�ǡ��Զ��ױ���������ɺͽṹ��ͼ������������£�
2014��4��2�ա�4�շ������廪ѧ��PX����������ս���������ڰٶȰٿ���������PX��Ϊ���Ͷ��������ȷ����������˽�ʾ��������ʵ�Ŀ�ѧ̬�ȣ�PX�ǡ��Զ��ױ���������ɺͽṹ��ͼ������������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com