
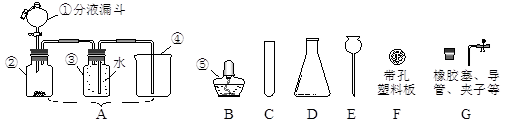
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������9��ѧʽ�����ϼۣ����� ���ͣ�������
��2011��㶫���죬24�⣩ý����ձ����������ϲ��ַ�������ܻ�����Ⱦ�����ڽӴ��ܻ���������ѪҺϵͳ����ֳϵͳ�����У��ܻ�����DMP������ʽΪC10H10O4����
��1��DMP����Է�������Ϊ_______��
��2��DMP������C��H��O ����Ԫ�ص�������Ϊ____________��
��3��DMP��������Ԫ�ص���������Ϊ�������ȷ��0.01��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������3���� ���ͣ������
��2011��㶫���죬19�⣩����ͼ��װ�������ر�ֹˮ�У�ͨ��ʹ����ȼ�ա���ش��������⣺

��1��ȼ�յ������� ��
��2������ȼ��һ��ʱ����Զ�Ϩ���ˣ�����Ϊԭ���� ��
��3����ȴ���ɿ�ֹˮ�У���۲쵽������Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������24�кͷ�Ӧ����Ӧ�ã�������Һ�����ȣ� ���ͣ������
��2011��㶫���죬18�⣩�������ų��ķ�ˮ�к��������������ƺ������ƣ����һ������ų��ķ�ˮ��Ϻ����Һ��pH=7����ֻ�����Ȼ���һ�����ʡ�����գ�
��1���һ������ķ�ˮ�к��е������ǣ��ѧʽ�� ��
��2��д������1����Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com