





| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����ʳƷ������������Ұ�����ʳ����ijɷ���þ�ۡ����ۡ��Ȼ��ơ�ʹ��ʱ����ˮ��þ��ˮ��Ӧ������������������18�桢������ѹ�Ļ����жԸò�Ʒ��ʵ���о�����������ʵ�鷽���ش��й����⣨�������Ȼ��ƶ�ˮ�е��Ӱ�죩��
ʵ��1��
��һ��������þ�������ۺ��Ȼ��Ƽ���ʢ��100 mLˮ�ĸ��������У��������裬ÿ50 S��¼һ���¶ȣ�����ͼ������a��
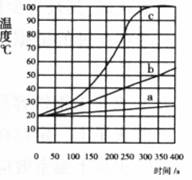
����ͬ������þ������100�ݴ������þ���ظ�����ʵ�飬����ͼ������b��
������ͬ������þ�۴������þ���ظ�����ʵ�飬��ͼ��������c��
��1���������仯�Ƕȿ����ñ仯���� ��ת��Ϊ �ܡ�
��2���۲���ͼ�����п��Է���Ӱ��þ��ˮ��Ӧ���ʵ������� ��
ʵ��2��
��2.40gþ�ۺ�����Ϊ28.00g�����ۻ�ϣ�����ʢ��100 mLˮ�ĸ��������У����Ͻ��衣�Ȼ���������ͬʱ���¶ȱ仯�������ͼ��ʾ��
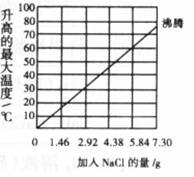
��3��ʵ��2�У���NaCl���� ����7.30gʱ��ʵ�鲻������ԭ���� ��
A����������NaCl�������ӷ�Ӧ����
B����������NaCl�ή�ͷ�Ӧ����
C���Ѵﵽˮ�ķе㣬�¶Ȳ������б仯
D��������������������ˮ���¶�
��4�������ʵ��2�м�������Ϊ3.65g NaCl���������¶���ߴ�Լ�� ��
��5��ijͬѧ�����������������ʳƷ�����������䷽������ݱ���ʵ���о��Ľ��ۣ��ж�������������� ��
A��2.40gþ�ۡ�7.30gNaCl��28.00g����
B��2.40gþ����7.30gNaCl��28.00g����
C��2.40gþ�ۡ�8.76gNaCl��28.00g����
D��2.40gþ����8.76gNaCl��28.00g����
�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�꽭��ʡ�������п���ѧ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ר��ͻ��ѵ����ʵ����̽���������棩 ���ͣ������


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com