

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
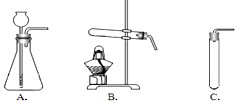
 ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
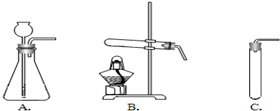
 ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����| ����ͷ��� | ���� | ���� |
| ȡ7.2g��ɫ���壬�����ձ��У������м���������ϡ���ᣬ��ֽ��裬���ã� | ���ޱ仯���� | ֤����ɫ������ Cu Cu �� |
| �� ��Һ����ɫ ��Һ����ɫ �� |
֤����ɫ����϶����� Cu2O Cu2O �� | |
| ȡ������Ӧ�����Һ���ˣ�ϴ�ӡ�����ͳ������ù�������Ϊ6.8g�� | �u | ȷ�Ϻ�ɫ������ Cu��Cu2O�Ļ���� Cu��Cu2O�Ļ���� �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com