
��ѧ������Ȥ�С���ͬѧ��ij����������������������ɷ�����ȡ������Ʒ��������ϡ
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ������ | ϡ��������� | ������Ʒ������ | ��Ӧ���ձ��л��������� |
| 200 g | 14.5 g | 214.0 g |
�������ձ��з�Ӧ�����������е����ʲ����ᷴӦ��Ҳ������ˮ������ʵ���������±������йط��Ļ�ѧ����ʽΪ��Fe �� 2HCl= FeCl2 �� H2������1�����������غ㶨�ɿ�֪����Ӧ�����ɵ�����������Ϊ_______ g����2�������������Ʒ��������������
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б仯�����ڻ�ѧ�仯���ǣ�������
A��ľ�����ɿ� B������ե��֭  C����ѩ����ˮ D��������ɾ�
C����ѩ����ˮ D��������ɾ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������߲��ų��õ�һ�ָ�Ч��ȫ��������������������ɱ�ʾΪRO2����Ҫ��������ˮ������ʵ���ø���������R��O��������Ϊ71��64����RO2�Ļ�ѧʽΪ��������
A��CO2 B��NO2 C��SO2 D��ClO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ⶪ���Ͼɸɵ�ػ���ɻ�����Ⱦ�����Ͼɸɵ���е��������ʶ��ǿ��Ի������õġ��������ͼ�ش��������⣺
(1)ͼʾ�����ڷǽ������ʵ���________���������������__________��(д��ѧʽ)
( 2)��пƤϴ�����������ȡ��������п��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��________________________________��
2)��пƤϴ�����������ȡ��������п��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��________________________________��
(3)ȡ�ɵ���ڵ����������ˮ�����ˣ��õ�A����Һ�ͺ�ɫ��������պ�ɫ�������MnO2��
��MnO2��ʵ������ȡ�����������____________���ã�
�ڽ�A����Һ��Ϊ���ݣ�һ�ݼ����ռ���Һ�����Ȳ���һ�ִ̼�����ζ�����壬����ʹʪ��ĺ�ɫʯ����ֽ����ɫ����֪������Ļ�ѧʽΪ______________����һ�ݼ���AgNO3��Һ��������ɫ�������μ�ϡHNO3�������ܽ⣬�����֪A���ʵĻ�ѧʽΪ__________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1911����������ѧ��¬ɪ������һ��������ġ������ȵ��Ӵ�ö�ĸ����˶��Ħ����Ӻ����ʱ���֣��ٴ�����������ܴ������ı�ԭ�����˶������ٲ��֦����Ӹı�ԭ���ķ�����ƫת���ۼ�������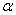 ���ӱ������˻������ɴ������Ƴ�����ԭ���ڲ��ṹ����Ϣ��
���ӱ������˻������ɴ������Ƴ�����ԭ���ڲ��ṹ����Ϣ��
A. ԭ�Ӻ������С
B. ԭ�Ӻ˴�����
C. ԭ���ڲ��С��ܴĿռ�
D. ԭ��ֻ��һ��ԭ�Ӻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Mg(OH)2�ֽ��������������������µ�MgO��ˮ������������һ���ʣ��ɽ�Mg(OH)2���ӵ���ȼ�Բ���������ȼ��������Mg(OH)2������ȼ���õ���������������
A��Mg(OH)2�ֽ�Ҫ���ȣ������˿�ȼ����Ż��
B��Mg(OH)2�ֽ��ܹ����£�ʹ��ȼ�ﲻ�״ﵽ�Ż��
C��Mg(OH)2�ֽ����ɵ�MgO�����ڿ�ȼ����棬�����˿���
D��Mg(OH)2�ֽ����ɴ���ˮ���������Ϳ�ȼ����Χ������Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
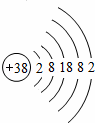
�ݡ���Ȼ�����ʱ�������ѧ��������Ƴ�һ�����ȣ�Sr�����Ӱڵ��ӣ������������ȷ���ӣ��ȵ�ԭ�ӽṹʾ��ͼ��ͼ��ʾ��
��1�� ��������Ԫ�أ���������ǽ���������
��������Ԫ�أ���������ǽ���������
��2����ԭ�ӵ�������Ϊ����������������������ӣ������������ӣ���á���ʧ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com