
| ||
| ||
| ||
| 100 |
| x |
| 44 |
| 17.6g |
| 40g |
| 50g |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
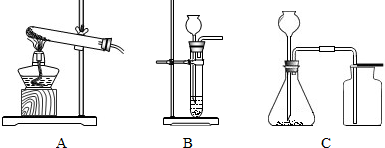
| A�����Թ��м���5mL5%�Ĺ���������Һ���������ǵ�ľ�������Թܣ�ľ����ȼ |
| B����ʢ��ˮ��С�ձ��м�������Ʒ�죬һ��ʱ�䣬�ձ��ڵ�Һ���Ϊ��ɫ |
| C��ľ̿��������ȼ�ղ�����ɫ���� |
| D��ʯ��ʯ������ϡ���ᷴӦʱ����������д����Ķ�����̼������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��Һ���¶�/�� | 75 | 65 | 50 | 35 | 20 |
| ��������M������/g | 0 | 0 | 2.0 | 4.5 | 8.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������ԭ�ӣ�
������ԭ�ӣ�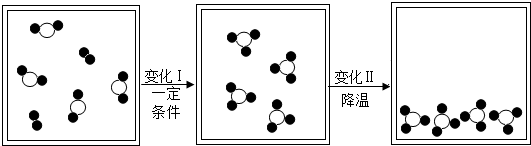
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ʵ������ | ���� |
| I��ȡ�����Ľ�����ĩ���Թ��У����������� | ����ȥ�� | |
| ���Թܾ��ã���ȥ�ϲ���Һ������������ϡ���� | ֤�������� | |
| ���Թܾ��ã���ȥ�ϲ���Һ����ˮ�����ϴ��ʣ����� | ʣ�������Ϻ�ɫ | ֤������ͭ |
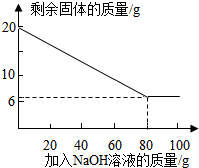
| ��NaOH��Һ�Ĵ��� | ��һ�� | �ڶ��� | ������ | �� |
| ʣ����������/g | 17.3 | n | 11.9 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | A | B | C | D |
| ����ˮ | ʳ�� | ���� | ʳ��ˮ | |
| pH | 10 | 3 | 9 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
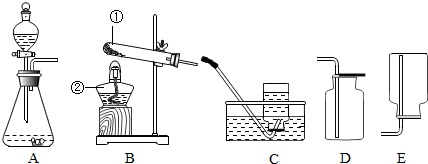
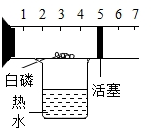 ��ͼ��һ�����п̶Ⱥͻ����ɻ����IJ��������������п����������İ��ף���������ʢ�з�ˮ���ձ��Ϸ�������ʵ�飮�����ʵ�鱨�棺
��ͼ��һ�����п̶Ⱥͻ����ɻ����IJ��������������п����������İ��ף���������ʢ�з�ˮ���ձ��Ϸ�������ʵ�飮�����ʵ�鱨�棺| ʵ��Ŀ�� | ʵ������ | ʵ����� | ||
| �ⶨ������ ��1����������� | �����Ż�ȼ�գ������� ��2�� | �����ijɷְ�������㣬��5��
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com