ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ���г��ֵ�Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ����������벻����Ч����

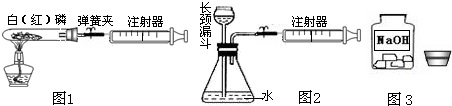
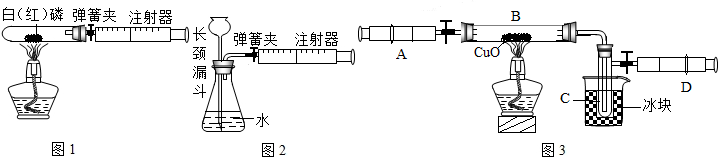
��1��ͼ1��50mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ��������50mLע�������������ȴ���20mL�̶ȴ���������ȼ�����ĵ����������
�������ټ��װ�õ������ԣ���װ��ҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ���������������Ϊ
����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ
mL�̶ȴ���ȡ����ֵ����˵���������������������ԼΪ
��
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ������ܹ۲쵽
��ѡ����ţ�����˵��װ�����������ã�
A��ע��������Һ�� B��ƿ��Һ������
C������©����Һ������ D������©���¶˹ܿڲ�������
��3��ijѧ��Ϊ�˲ⶨ������Ԫ���γɵ���̬������X����ɣ�������ͼ3��ʾ��ʵ�飮����ע����A�е�����X��������װ��CuO��Bװ�ã�ʹ֮��ȫ��Ӧ���õ����½����
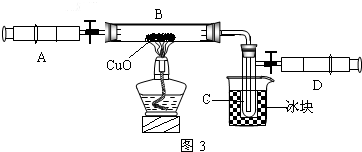
��ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g��
��C�����ռ��������ʵ���õ�H
2��O
2����D���ռ�������N
2��
��X����Ԫ�ص���������14��3���ʣ�
����C���ռ�����Һ�壬������
g��
������ʵ���п�����������
��
����B�з�Ӧ�Ļ�ѧ����ʽ��
��
��4��ȡ8gCuO��δ����Ҫ�����������20%��������Һ��ǡ����ȫ��Ӧ������д��������̣�






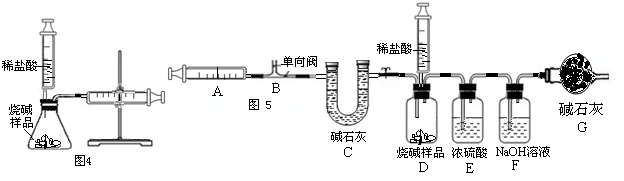



 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����