
£Ø5·Ö£©ĻÖÓŠĮ½ÖÖĻ”ČÜŅŗ£ŗ±ź¼ĒĪŖAµÄ0.0400%µÄĒāŃõ»ÆÄĘČÜŅŗ£»±ź¼ĒĪŖBµÄ0.365%µÄŃĪĖį”£¼ŁÉč±¾ĢāĖłÉę¼°µ½µÄø÷ÖÖĻ”ČÜŅŗµÄĆÜ¶Č¾ł½üĖĘĪŖ1.00g”¤mL-1£¬ĒŅĆæµĪČÜŅŗµÄĢå»ż½üĖĘĪŖ0. 05mL£¬ŹŌ½ā“šĻĀĮŠø÷Š”Ģā”£
£Ø1£©Ē”ŗĆĶźČ«ÖŠŗĶ20. 0gAČÜŅŗ£¬Šč¼ÓČėBČÜŅŗ¶ąÉŁæĖ£æ
£Ø2£©ŌŚŹ¢ÓŠ20. 0mLAČÜŅŗµÄ׶ŠĪĘæÖŠµĪ¼Ó2µĪ·ÓĢŖŹŌŅŗ£¬ŌŁĻņĘæÖŠ»ŗ»ŗµ¹ČĖ10. 0mLBČÜŅŗ£¬±ßµ¹±ßÕńµ“£¬³ä·Ö»ģŗĻŗóČÜŅŗ³ŹĪŽÉ«”£ČōČ”øĆĪŽÉ«»ģŗĻŅŗ3.00mLÓŚŅ»Ö§ŹŌ¹ÜÄŚ£¬ŌŁĻņŹŌ¹ÜÄŚµĪ¼Ó1µĪAČÜŅŗ£¬ŹŌĶعż¼ĘĖćĖµĆ÷“ĖŹ±ŹŌ¹ÜÄŚČÜŅŗ³ŹĻÖµÄŃÕÉ«”£
£Ø1£©2.00g£» £Ø2£©ČÜŅŗĻŌĪŽÉ«
½āĪöŹŌĢā·ÖĪö£ŗŅŃÖŖĮæ£ŗĒāŃõ»ÆÄĘ£ŗ20.0g”Į0.0400%=0.008g£» 0.365%µÄŃĪĖį£»10. 0mLBČÜŅŗÖŠĀČ»ÆĒāµÄÖŹĮ棻10. 0mLBČÜŅŗ£»»ģŗĻŅŗ3.00mL£»Ī“ÖŖĮæ£ŗ£Øl)Ē”ŗĆĶźČ«ÖŠŗĶ20. 0gAČÜŅŗ£¬Šč¼ÓČėBČÜŅŗµÄÖŹĮ棻£Ø2£©ŹŌ¹ÜÄŚČÜŅŗµÄŃÕÉ«£»
½ā£ŗ£Ø1£©Ē”ŗĆĶźČ«ÖŠŗĶ20. 0gAČÜŅŗ£¬Šč¼ÓČėBČÜŅŗµÄÖŹĮæĪŖx
HCl + NaOH==NaCl+H2O
36.5 40
0.365%x 0.008g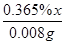 =
=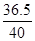
x=2.00g
£Ø2£©20. 0mLĒāŃõ»ÆÄĘČÜŅŗÖŠŗ¬ÓŠĒāŃõ»ÆÄĘÖŹĮæĪŖ8g£»ŅņĪŖĒ”ŗĆĶźČ«ÖŠŗĶ20. 0gAČÜŅŗ£¬Šč0.365%µÄŃĪĖįµÄÖŹĮæĪŖ2.00g£¬3mLŃĪĖįÖŠĀČ»ÆĒāµÄÖŹĮæĪŖ£ŗ3mL”¤30mL-1”Į8g”Į0.365%=2.92g”Į10-3g£»
Éč1µĪAČÜŅŗÖŠµÄĒāŃõ»ÆÄĘÄÜÖŠŗĶ1ĀČ»ÆĒāµÄÖŹĮæĪŖy£¬
HCl + NaOH==NaCl+H2O
36.5 40
y 0.05g”Į0.04%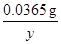 =
=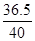
y-1.825g”Į10-3g<2.92g”Į10-3g;
ĖłŅŌŃĪĖįÓŠŹ£Óą£¬ČÜŅŗĻŌĪŽÉ«”£
æ¼µć£ŗ »Æѧ·½³ĢŹ½¼ĘĖć£»Źż¾ŻµÄ“¦Ąķ£»Ėį¼īÖøŹ¾¼ĮµÄŃÕÉ«±ä»Æ


 æģĄÖŠ”²©Źæ¹®¹ĢÓėĢįøßĻµĮŠ“š°ø
æģĄÖŠ”²©Źæ¹®¹ĢÓėĢįøßĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
Č”Ņ»¶ØĮæFe2O3ÓėAl2O3µÄ»ģŗĻĪļ£¬¼ÓČėŗ¬ČÜÖŹ9.8gµÄĻ”ĮņĖį£¬Ē”ŗĆĶźČ«·“Ó¦£®Ō»ģŗĻĪļÖŠŃõŌŖĖŲµÄÖŹĮæŹĒ£Ø””””£©
| A£®0.8g | B£®1.6g | C£®3.2g | D£®6.4g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ŠĖȤŠ”×éÓū²ā¶ØijĪ“ÖŖÅضČBa£ØOH£©2ČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż£¬ĒėÄćŗĶĖūĆĒŅ»ĘšĶź³ÉŹµŃé²¢»Ų“šÓŠ¹ŲĪŹĢā£®
£Ø1£©ÅäÖĘ30g 10%µÄNa2CO3ČÜŅŗ£®
²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘČ””¢ĮæČ””¢”” ”””¢×ŖŅĘ£®ĮæČ”Ė®Ź±Ó¦Ń”ÓĆ¹ęøńĪŖ”” ””mL£Ø“Ó10”¢50”¢100֊єȔ£©µÄĮæĶ²£®×īŗó½«Ģ¼ĖįÄĘČÜŅŗ×ŖŅʵ½ŹŌ¼ĮĘæÖŠ£¬ĢłÉĻ±źĒ©±øÓĆ£¬ŌŚ±źĒ©ÉĻæÉŅŌ²»±Ų×¢Ć÷µÄŹĒ”” £ØĢīŠņŗÅ£©£®
A£®30g B£®10% C£®Na2CO3ČÜŅŗ
£Ø2£©²ā¶ØĪ“ÖŖÅضČBa£ØOH£©2£¬ČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż£®
Č”50g Ba£ØOH£©2ČÜŅŗ£¬ÖšµĪµĪ¼ÓÉĻŹöNa2CO3ČÜŅŗµÄ¹ż³ĢÖŠ£¬²āµĆ²śÉś³ĮµķÓė¼ÓČėNa2CO3ČÜŅŗµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®
¢ŁĒ”ŗĆĶźČ«·“Ó¦Ź±£¬ĻūŗÄNa2CO3ČÜŅŗµÄÖŹĮæĪŖ”” ””g£®
¢ŚĒėĶعż¼ĘĖćČ·¶ØBa£ØOH£©2ČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹż£®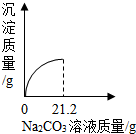
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
»ĘĶŹĒĶŗĶŠæµÄŗĻ½š£¬Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ŌŚ²ā¶Ø»ĘĶÖŠĶµÄŗ¬ĮæŹ±£¬Č”»ĘĶѳʷ40g£¬·ÅČėÉÕ±ÖŠ£¬ĻņĘäÖŠ¼ÓČė200gĻ”ĮņĖį£¬Ē”ŗĆĶźČ«·“Ó¦£¬·“Ó¦ŗóÉÕ±ÖŠŹ£ÓąĪļµÄ×ÜÖŹĮæĪŖ239.6g£¬Ēė¼ĘĖć
¢Ł²śÉśĒāĘųµÄÖŹĮæŹĒ £»
¢Ś²ĪÓė·“Ó¦µÄĻ”ĮņĖįµÄÖŹĮæŹĒ £»
¢Ū»ĘĶÖŠĶµÄÖŹĮæ·ÖŹż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø13·Ö£©Ņ½Ń§ÉĻ¾³£ÓĆĮņĖįŃĒĢśĢĒŅĀʬøųʶŃŖ»¼Õß²¹Ģś”£Ä³ŠĖȤŠ”×éµÄĶ¬Ń§¶ŌĢĒŅĀʬ֊ĮņĖįŃĒĢś¾§ĢåµÄÖʱøŗĶ×é³É²śÉśĮĖŠĖȤ²¢¶ŌĘä½ųŠŠĢ½¾æ”£
Ģ½¾æ¢ń£ŗĄūÓĆŹµŃéŹŅµÄ·ĻĖ®»ŲŹÕĶ”¢ÖʱøĮņĖįŃĒĢś¾§Ģ唣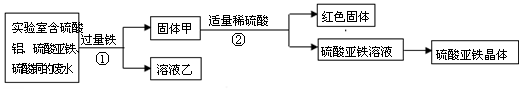
£Ø1£©²½Öč¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø2£©¹ĢĢå¼×ÖŠŗ¬ÓŠµÄĪļÖŹŹĒ (Ģī»ÆѧŹ½£¬ĻĀĶ¬) £»ŗģÉ«¹ĢĢåŹĒ £»ČÜŅŗŅŅÖŠµÄČÜÖŹŹĒ ”£
Ģ½¾æ¢ņ£ŗĮņĖįŃĒĢś¾§Ģå£ØFeSO4?xH2O£©µÄ»ÆѧŹ½”£
”¾²éŌÄ׏ĮĻ”æ
£Ø1£©ĪŽĖ®ĮņĖįĶ·ŪÄ©ÓöĖ®»į±ä³ÉĄ¶É«µÄĮņĖįĶ¾§Ģ唣
£Ø2£©ĮņĖįŃĒĢś¾§Ģå¼ÓČČŹ±£¬ĻČŹ§Č„½į¾§Ė®£¬øßĪĀ»į¼ĢŠų·Ö½ā²śÉś½šŹōŃõ»ÆĪļŗĶĘųĢ¬·Ē½šŹōŃõ»ÆĪļ”£
”¾½ųŠŠŹµŃé”æ
øĆŠĖȤŠ”×éĶ¬Ń§³ĘČ”2.78gĮņĖįŃĒĢś¾§Ģå£ØFeSO4?xH2O£©ŃłĘ·°“Ķ¼1×°ÖĆøßĪĀ¼ÓČČ£¬Ź¹ĘäĶźČ«·Ö½ā£¬²¢¶ŌĖłµĆ²śĪļ½ųŠŠ·ÖĪö£¬²¢ĄūÓĆSDTQ600ČČ·ÖĪöŅĒ¶ŌĮņĖįŃĒĢś¾§ĢåČČ·Ö½ā»ńµĆĻą¹ŲŹż¾Ż£¬»ęÖĘ³ÉĶ¼2ĖłŹ¾¹ŲĻµĶ¼”£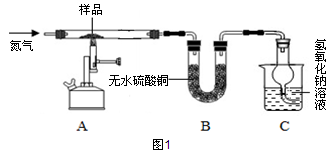
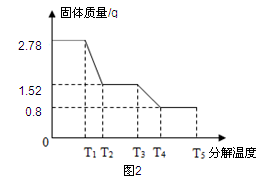
”¾Źż¾Ż·ÖĪö”æ
£Ø1£©Ķ¼1×°ÖĆBÖŠĪŽĖ®ĮņĖįĶ·ŪÄ©±äĄ¶£¬ĖµĆ÷²śĪļÖŠÓŠ £¬øĆĪļÖŹµÄÖŹĮæĪŖ g”£
£Ø2£©T3”ꏱ£¬·¢ÉśµÄ»Æѧ·“Ó¦ĪŖ£ŗ2FeSO4øßĪĀFe2O3+X”ü+SO3”ü£¬ĘäÖŠXµÄ»ÆѧŹ½
ӣ
£Ø3£©×°ÖĆCÖŠĒāŃõ»ÆÄĘČÜŅŗµÄ×÷ÓĆŹĒ ”£
£Ø4£©ŅŃÖŖFeSO4?xH2O ”÷FeSO4 + xH2O£»
¼ĘĖćFeSO4?xH2OÖŠµÄx”££ØŠ“³ö¼ĘĖć¹ż³Ģ£©
”¾½»Į÷ĢÖĀŪ”æŹµŃéÖŠŅŖ³ÖŠųĶØČėµŖĘų£¬·ńŌņ²ā³öµÄxÖµ»į £ØĢīĘ«“ó”¢Ę«Š”»ņ²»±ä£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ŹÆ»ŅŹÆѳʷµÄÖ÷ŅŖ³É·ÖŹĒCaCO3£ØŅŃÖŖĘäĖüŌÓÖŹ²»ÓėŃĪĖį·“Ó¦£©£®æĪĶāŠ”×éĶ¬Ń§½«50gŃĪĖį·Ö5“Ī¼ÓČėµ½20gøĆŹÆ»ŅŹÆѳʷ֊£¬µĆµ½ČēĻĀ²æ·ÖŹż¾ŻŗĶĶ¼Ļó£ŗ
| “ĪŹż | µŚ1“Ī | µŚ2“Ī | µŚ3“Ī |
| ¼ÓČėŃĪĖįµÄÖŹĮæ/g | 10 | 10 | 10 |
| Ź£Óą¹ĢĢåµÄÖŹĮæ/g | 16 | 12 | 8 |
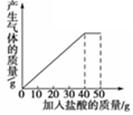
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ĪŖĮĖĢ½¾æŹÆ»ŅŹÆÓėĻ”ŃĪĖį·“Ó¦Öʱø¶žŃõ»ÆĢ¼ŗóµÄ·ĻŅŗÖŠµÄČÜÖŹ³É·Ö£¬½«·ĻŅŗ¹żĀĖ£¬Č”ĀĖŅŗ20æĖÓŚÉÕ±ÖŠ£¬ŌŚ²»¶ĻÕńµ“µÄĢõ¼žĻĀ£¬ĻņĘäÖŠµĪ¼ÓÖŹĮæ·ÖŹżĪŖ10.6£„µÄĢ¼ĖįÄĘČÜŅŗÖ±ÖĮ¹żĮ棬ӊ¹ŲµÄ±ä»ÆČēĶ¼ĖłŹ¾£ŗøł¾ŻĢāÖŠÓŠ¹ŲŠÅĻ¢ŗĶĶ¼Ļń·ÖĪö»Ų“šĻĀĮŠĪŹĢā£ŗ
Ō·ĻŅŗÖŠµÄČÜÖŹ³É·ÖÓŠ ÓĆ»ÆѧŹ½»Ų“š£©”£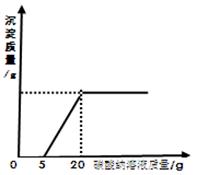
(2)Aµć“¦ČÜŅŗÖŠČÜÖŹµÄÖŹĮæŹĒ¶ąÉŁæĖ£æ(Ķعż¼ĘĖć»Ų“š)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ĻÖÓŠŗ¬HClŗĶCuCl2µÄ»ģŗĻČÜŅŗ50g£¬ĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČėČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮæÓė¼ÓČėNaOHČÜŅŗµÄÖŹĮæ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£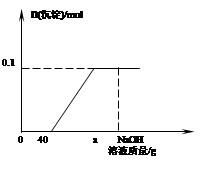
¢Ł Š“³öÓŠ¹ŲµÄ·“Ó¦·½³ĢŹ½ ¢Ó £¬ ¢Ō £»
¢Ś µ±¼ÓČėNaOHČÜŅŗÖŹĮæĪŖagŹ±£¬ČÜŅŗÖŠµÄČÜÖŹŹĒ ¢Õ £»
¢Ū Ēó»ģŗĻČÜŅŗÖŠCuCl2µÄÖŹĮæ·ÖŹż ¢Ö ”£
£ØĒėøł¾Ż»Æѧ·½³ĢŹ½¼ĘĖć£©
¢Ü ĒóĒ”ŗĆĶźČ«·“Ó¦Ź±£¬ĻūŗÄNaOHČÜŅŗµÄ×ÜÖŹĮæ ¢× ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
Š”Ć÷ŌŚĄĻŹ¦µÄÖøµ¼ĻĀ“ÖĀŌ²āĮæŅ»ĘæĻ”ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹż£¬¾ßĢå²½ÖčČēĻĀ£ŗ
²½ÖčŅ»£ŗÅäÖĘČÜÖŹÖŹĮæ·ÖŹżŌ¼ĪŖ1%µÄĒāŃõ»ÆÄĘČÜŅŗ”£
²½Ö趞£ŗĻņ10æĖ“ż²āĻ”ŃĪĖįÖŠÖšµĪµĪČėÉĻŹöĒāŃõ»ÆÄĘČÜŅŗ£¬²¢Ź¹
ÓĆpH¼Ę¼ĒĀ¼ČÜŅŗµÄpH±ä»ÆĒéæö£¬»ęÖĘĶ¼ĻńČēĶ¼”£
£Ø1£©ŌŚ”°²½ÖčŅ»”±ÖŠÓŠ¼ĘĖć”¢ ”¢ČܽāČżøö¾ßĢå²½Öč”£
£Ø2£©ŌŚĶłĻ”ŃĪĖįÖŠÖš½„µĪČėĒāŃõ»ÆÄĘČÜŅŗµÄ¹ż³ĢÖŠ£¬ŌŚaµćµÄČÜŅŗÖŠ
µÄĪ¢Į£ÓŠ ”££ØÓĆ»Æѧ·ūŗűķŹ¾£©
£Ø3£©Ēė½įŗĻÓŅĶ¼£¬¼ĘĖć¢ŁøĆĻ”ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹż£»¢ŚbµćŹ±ČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż”££Ø½į¹ū¾«Č·µ½0.1%£©£ØŠ“³ö¼ĘĖć¹ż³Ģ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com