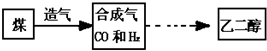
�ҹ����нϷḻ��ú̿��Դ��ú��ȡ�Ҷ����IJ�ҵ����չ��ú�ۺ����õķ���ú��ȡ�Ҷ���������ʾ��ͼΪ��

��1��ú����______ �������������������������Դ��
��2���ϳ������л�ԭ�ԣ�д��CO��ԭ����ͭ�Ļ�ѧ����ʽ��______���û�ѧ��Ӧ�����ڻ�ԭ����������______��
��3���ϳ���ȡ�Ҷ����Ļ�ѧ��Ӧ����ʽΪ��2CO+3H
2C
2H
6O
2���û�ѧ��Ӧ�Ļ���������______���ӵ���ʧ���ĽǶȷ������÷�ӦҲ��������ԭ��Ӧ������������������������______��
��4�����ݶԣ�2���루3���ķ��������CO��������ʲô�µ���ʶ��______��
��5���ϳ�����CO��H
2���ڲ�ͬ�����������£����Ժϳɲ�ͬ�����ʣ����úϳ���Ϊԭ�ϲ����ܵõ���������______ ������ĸ��ţ���
a�����ᣨHOOCCOOH�� b���״���CH
3OH�� c������[CO��NH
2��
2]��




 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д�


 C2H6O2���û�ѧ��Ӧ�Ļ���������______���ӵ���ʧ���ĽǶȷ������÷�ӦҲ��������ԭ��Ӧ������������������������______��
C2H6O2���û�ѧ��Ӧ�Ļ���������______���ӵ���ʧ���ĽǶȷ������÷�ӦҲ��������ԭ��Ӧ������������������������______��