
��2011�긣�����ң�12�⣩���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ�������ͼ��ʾ���ش��������⣺

��1�������������̵���ʾ��ͼ���£����Ʊ��ϳ����Ļ�ѧ����ʽΪ ��

��2���ϳ����л�ԭ�ԣ�������ұ����������д���ϳ�����Fe2O3��Ӧ��һ����ѧ����ʽ�� ��
��3���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ����úϳ���Ϊԭ�ϲ����ܵõ��������� ������ĸ��ţ���
A�����ᣨHOOCCOOH�� B���״���CH3OH�� C����[CO(NH2)2]
��4����úֱ����ȼ�ϵ���ú��ȡ�Ҷ�������õ�����ʾ�� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������11 ��ѧ����ʽ����д�����ֻ������ͣ� ���ͣ������
��2011�긣�����ң�12�⣩���ù�ҵú���Ҷ�������Ҫ�Ļ���ԭ�ϣ�������еͳɱ������ܺġ����ŷŵ��ص�����зdz�������ǰ�������Ʊ�������ͼ��ʾ���ش��������⣺
��1�������������̵���ʾ��ͼ���£����Ʊ��ϳ����Ļ�ѧ����ʽΪ ��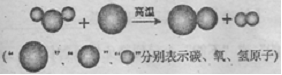
��2���ϳ����л�ԭ�ԣ�������ұ����������д���ϳ�����Fe2O3��Ӧ��һ����ѧ����ʽ�� ��
��3���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ����úϳ���Ϊԭ�ϲ����ܵõ��������� ������ĸ��ţ���
A�����ᣨHOOCCOOH�� B���״���CH3OH�� C����[CO(NH2)2]
��4����úֱ����ȼ�ϵ���ú��ȡ�Ҷ�������õ�����ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������25 �Σ����� ���ͣ��ƶ���
��2011�긣�����ң�15�⣩��ͬѧ���й��ε�֪ʶ���������µ�������
��1�������ε����������� ˳����������ġ�
��2������������������ƶ�����ijЩ��ͬ�Ļ�ѧ���ʣ��綼����BaCl2��Һ��Ӧ�������ᱵ�������÷�Ӧ�Ļ�ѧ����ʽΪ ����ѡ����һ���μ��ɣ���
��3����ͬѧͨ���������ϣ��������෴Ӧ���������������ɡ�������Щ֪ʶ�����ϵ��������ͼ���뽫��ͼ��������a ��b ��
��4����ͬѧ���������ͼָ���������ʵ��Ʊ�����д��2���й������������ɵĻ�ѧ��Ӧ����ʽ��
�� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п����������ר���� ������Ʊ� ���ͣ������
��2011�긣�����ң�16�⣩ij��ȤС����������װ�ý���O2��CO2ʵ�����Ʒ����й����ʵ��о���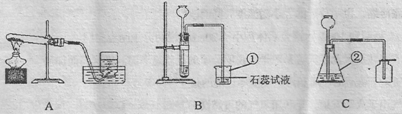
��1��д�����б�����������ƣ��� �� ��
��2����ͬѧ��ϡ����ʹ���ʯ��Bװ���з�Ӧ���۲쵽�ձ�����ɫʯ����Һ ��������
��3����ͬѧҪ��KMnO4��������ȡO2��Ӧѡ����ͼ�е� �����ţ����÷�Ӧ�Ļ�ѧ����ʽ�� ����ͬѧ�����ռ�����������м��飬��д������������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com