
| ���� | �Ȼ��ơ������ |
| ��Ԫ�غ��� | 20-30mg/Kgʳ�� |
| ������ | 18���� |
| ʹ�÷��� | ��ʱ������ |
| ����ָ�� | �ܹ⡢���ȡ��ܷ⡢���� |
| 0.25mg |
| 30mg/Kg |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺
���µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
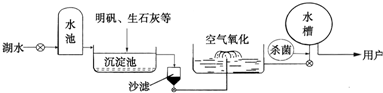
 ��2012?Ϋ�������µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺
��2012?Ϋ�������µĿ������ྻ��ˮ�������Ӫ���������彡��ϢϢ��أ�����������ѧ��ѧ֪ʶ�ش��������⣺
| ||
| ʳ�� | ��� | ���� | ������ | ţ�� |
| �����ʺ��� | 10% | 14% | 20% | 3% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�갲��ʡ�Ϸ���®���ؾ��꼶��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��Ԫ�������彡��ϢϢ��أ���ά�������״�����������������Ԫ�ء�������ȱ��ʱ�ͻ��״���״��׳ơ����Ӳ�����
��1�����Ԫ�ط���Ϊ ������ �������Ԫ�ء��ǽ���Ԫ�ء�����
��2����ͼ��ijƷ��ʳ�ΰ�װ����ij����ÿ���貹���Ԫ�ص�����Ϊ0.25mg����������ȡ��ȫ�����ڸ�Ʒ��ʳ�Σ��Լ���ó���ÿ����������ʳ�ε�����Ϊ����Kg��
|
���� |
�Ȼ��ơ������ |
|
��Ԫ�غ��� |
20��30mg/Kgʳ�� |
|
������ |
18���� |
|
ʹ�÷��� |
��ʱ������ |
|
����ָ�� |
�ܹ⡢���ȡ��ܷ⡢���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com