£Ø2009?ŗ£µķĒų¶žÄ££©øł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£®

£Ø1£©ŅĒĘ÷aµÄĆū³Ę
ŹŌ¹Ü
ŹŌ¹Ü
£¬A×°ÖĆÖŠ£¬¼ÓČė»ņĶØČėĻĀĮŠĪļÖŹ£¬×ĻÉ«ŹÆČļ±äŗģÉ«µÄŹĒ£ØĢīŠņŗÅ£©
¢Ł¢Ū
¢Ł¢Ū
£®
¢ŁĶØČė¶žŃõ»ÆĢ¼ ¢ŚĶØČėĒāĘų ¢Ū¼ÓČėŃĪĖį
¢Ü¼ÓČė°±Ė® ¢Ż¼ÓČėĀČ»ÆÄĘ ¢ŽĖ®
£Ø2£©B×°ÖĆÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£¬ÓėÕż¼«ĻąĮ¬µÄŹŌ¹ÜÖŠ²śÉśĘųĢåµÄ¼ģŃé·½·ØĪŖ
½«“ų»šŠĒµÄľĢõææ½ü¹ÜæŚ£¬¹Ū²ģµ½Ä¾Ģõø“Č¼
½«“ų»šŠĒµÄľĢõææ½ü¹ÜæŚ£¬¹Ū²ģµ½Ä¾Ģõø“Č¼
£®
£Ø3£©C×°ÖĆÖŠ£¬Č¼ÉÕ³×ÖŠµćČ¼µÄŗģĮ×£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£¬
µ±“ņæŖÖ¹Ė®¼Š£¬¼ÆĘųĘæÖŠ½ųČėŅŗĢåµÄĢå»ż£¬Ō¼Õ¼¼ÆĘųĘæĢå»żµÄ1/5£¬ŌņĖµĆ÷æÕĘųÖŠ
£®
£Ø4£©ČōC×°ÖĆµÄ¼ÆĘųĘæÖŠŹĒ¶žŃõ»ÆĢ¼£¬²åČėµćČ¼µÄĆ¾Ģõ£¬¹Ū²ģµ½µÄĻÖĻóŹĒĆ¾Ģõ¼ĢŠųČ¼ÉÕ£¬·“Ó¦ĶźČ«ŗ󣬼ÆĘųĘæµ×²æÓŠ°×É«ŗĶŗŚÉ«¹ĢĢ壬ĶĘ²āĆ¾Óė¶žŃõ»ÆĢ¼·“Ó¦µÄ»Æѧ·½³ĢŹ½
£®


 2H2”ü+O2”ü£¬½«“ų»šŠĒµÄľĢõææ½ü¹ÜæŚ£¬¹Ū²ģµ½Ä¾Ģõø“Č¼£»
2H2”ü+O2”ü£¬½«“ų»šŠĒµÄľĢõææ½ü¹ÜæŚ£¬¹Ū²ģµ½Ä¾Ģõø“Č¼£» 2P2O5£¬ŃõĘųµÄĢå»żÕ¼
2P2O5£¬ŃõĘųµÄĢå»żÕ¼ £»
£» 2MgO+C£®
2MgO+C£®


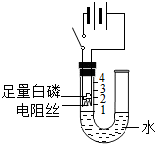 18”¢ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾ææÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬Éč¼ĘĮĖČēÓŅĶ¼ĖłŹ¾×°ÖĆ£®Ēėøł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£ŗ
18”¢ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾ææÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬Éč¼ĘĮĖČēÓŅĶ¼ĖłŹ¾×°ÖĆ£®Ēėøł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£ŗ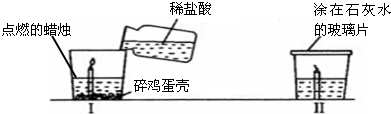

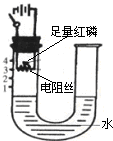 ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾ææÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾×°ÖĆ£®Ēėøł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£ŗ
ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾ææÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾×°ÖĆ£®Ēėøł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£ŗ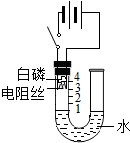 ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾ææÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾×°ÖĆ£®ĘäÖŠUŠĶ¹Ü×ó²ąČŻĘ÷±ŚÉĻ±źÓŠæĢ¶Č£¬µē×čĖæĶصēŗóÄÜ·¢ČČ£¬ÓŅ²ą²£Į§¹Ü³ØæŚ£®Ēėøł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£ŗ
ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾ææÕĘųÖŠŃõĘųµÄĢå»ż·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾×°ÖĆ£®ĘäÖŠUŠĶ¹Ü×ó²ąČŻĘ÷±ŚÉĻ±źÓŠæĢ¶Č£¬µē×čĖæĶصēŗóÄÜ·¢ČČ£¬ÓŅ²ą²£Į§¹Ü³ØæŚ£®Ēėøł¾ŻĶ¼Ź¾ŹµŃé»Ų“šĻĀĮŠĪŹĢā£ŗ