
| 序号 | 加入稀盐酸的质量(g) | 剩余固体的质量(g) |
| 第1次 | 10 | 5.5 |
| 第2次 | 10 | M |
| 第3次 | 10 | 1.2 |
| 第4次 | 10 | 1.2 |
分析 (1)根据碳酸钙和盐酸反应生成氯化钙、水和二氧化碳进行分析;
(2)由表中数据可知,第一次碳酸钙反应了2.5g,第三次剩余1.2g,说明第二次碳酸钙反应了2.5g;从而得出m的值;
(3)由表中数据可知,第一次碳酸钙反应了2.5g,第三次剩余1.2g,说明第二次碳酸钙反应了2.5g;第三次和第四次剩余物质都是1.2g,说明第三次后碳酸钙完全反应;
(4)根据参加反应的碳酸钙的质量,计算生成二氧化碳的质量;
(5)根据样品中碳酸钙的质量计算出杂质质量,所得不饱和溶液的质量=反应后固液混合物的质量-杂质质量+加入水的质量,溶质质量就是(2)中计算出的溶质(CaCl2)的质量,然后根据溶质质量分数公式计算即可;
(6)根据(2)中计算出的参加反应的HCl的质量计算出实验所用的稀盐酸的质量分数,根据溶质质量一定,利用溶质质量分数公式进行计算即可.
解答 解:(1)碳酸钙和盐酸反应生成氯化钙、水和二氧化碳,反应的化学方程式为:CaCO3+2HC1═CaC12+H2O+CO2↑;
(2)根据第一次10g盐酸消耗碳酸钙的质量为2.5g,第三次剩余固体成为了1.2g,说明第一次反应后还有碳酸钙,也就是说10g盐酸已经全部反应,只能消耗2.5g碳酸钙,再加10g盐酸,还能消耗2.5g,所以剩余固体:5.5g-2.5g=3g,所以m为3;
(3)了第三次假设这一次10g盐酸还能完全反应,最后剩余固体3g-2.5g=0.5g,结果剩余1.2g,说明盐酸没完全反应,剩余的都是杂质,即1.2g是杂质,碳酸钙质量为8g-1.2g=6.8g,样品中碳酸钙的质量分数是$\frac{8g-1.2g}{8g}$×100%=85%;
(4)设二氧化碳的质量为x,
CaCO3+2HCl═CaCl2+H2O+CO2↑,
100 44
6.8g x
$\frac{100}{6.8g}=\frac{44}{x}$
x=2.992g
第二次实验后,参加反应的碳酸钙质量为5g,设生成氯化钙质量为z,生成二氧化碳质量为m,稀盐酸的质量分数为n
CaCO3+2HCl═CaCl2+H2O+CO2↑,
100 73 111 44
5g n×20g z m
$\frac{100}{5g}$=$\frac{111}{z}$=$\frac{44}{m}$=$\frac{73}{n×20}$
z=5.55g
m=2.2g
n=18.25%
要将得到的滤液变成溶质质量分数为10%的溶液,应向溶液中加水x
$\frac{5.55g}{20g+5g-2.2g+x}$×100%=10%
x=32.7g;
(6)需要加水的质量是:40g-$\frac{40g×18.25%}{36.5%}$=20g.
故答案为:(1)CaCO3+2HC1═CaC12+H2O+CO2↑;
(2)3;
(3)85%;
(4)$\frac{100}{6.8g}$=$\frac{44}{x}$;
(5)32.7;
(6)20g.
点评 本题主要考查学生运用假设法和化学方程式进行计算和推断的能力,计算时要注意规范性和准确性.


 天天向上一本好卷系列答案
天天向上一本好卷系列答案科目:初中化学 来源: 题型:解答题
查看答案和解析>>
科目:初中化学 来源: 题型:填空题

查看答案和解析>>
科目:初中化学 来源: 题型:解答题
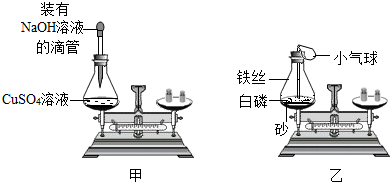
| 实验 | 化学反应的实验现象 | ②天平是否平衡 |
| 甲 | 有蓝色絮状沉淀出现 | |
| 乙 | 发出白光,放出热量,产生天平是否平衡大量白烟 |
查看答案和解析>>
科目:初中化学 来源: 题型:解答题
查看答案和解析>>
科目:初中化学 来源: 题型:解答题
查看答案和解析>>
科目:初中化学 来源: 题型:解答题
查看答案和解析>>
科目:初中化学 来源: 题型:选择题
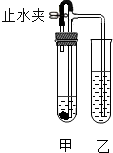 某同学用如图所示装置进行实验(图中铁架台等仪器均已略去).在甲试管中加入试剂后,塞紧橡皮塞,立即打开止水夹,乙试管中有气泡冒出;一段时间后关闭止水夹,乙试管中液面上升,溶液由澄清变浑浊.符合上述实验现象的甲、乙试管中应加入的试剂是( )
某同学用如图所示装置进行实验(图中铁架台等仪器均已略去).在甲试管中加入试剂后,塞紧橡皮塞,立即打开止水夹,乙试管中有气泡冒出;一段时间后关闭止水夹,乙试管中液面上升,溶液由澄清变浑浊.符合上述实验现象的甲、乙试管中应加入的试剂是( )| A | B | C | D | |
| 甲 | Zn、稀H2SO4 | Cu、稀H2SO4 | CaCO3、稀HCl | Na2CO3、稀H2SO4 |
| 乙 | BaCl2 | Ba(OH)2 | KNO3 | NaCl |
| A. | A | B. | B | C. | C | D. | D |
查看答案和解析>>
科目:初中化学 来源: 题型:填空题
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com