
��֪ij������Ʒ�к���Na2SO4��MgCl2��CaCl2�����ʡ�ʵ�����ᴿ�������£�
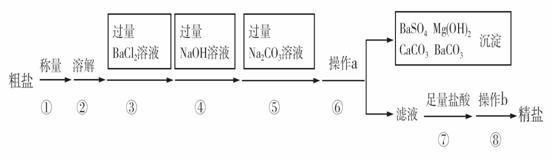
��1����������ƽ��������ʱ����ָ��ƫ���ұߣ����ʾ����������ȷѡ��Ĵ��룩 ��
A�������أ������� B�������ᣬ��Ʒ��
C�������أ���Ʒ�� D�������ᣬ������
��2���ڢܲ�����������Ӧ�Ļ�ѧ����ʽ�� ��
��3���ڢݲ�������Ŀ���� ��
��4���ڢ�����a�������� ���˲������У���������ĩ��Ҫ�����б����
��һ�ߡ�
��5���ڵڢ߲������У�����Һ�еμ����������Ŀ���� ��
��6���ڵڢಽ����ʱ��Ҫ�ò��������Ͻ��裬Ŀ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������������ء�
(1)����ͨ��ʳ���ȡ����Ӫ���ء�
������ʳ���У����ṩ���������ʵ��� ������ĸ��ţ���

A���� B�������� C��ţ��
��Ϊ�˷�ֹ�������ɣ�����ÿ�ձ��������㹻����________Ԫ�ء�
(2)ʳƷ��ȫ�����ܵ����ǹ�ע������ʳƷ���ж�������ʳ�õ��� ������ĸ��ţ���
A���������Ƶľ� B�������ͳ��IJ�
C����ȩ���ݵĺ���Ʒ D��ù��Ĵ��ס�����
(3)���ž��õķ�չ����Դ�뻷����Ϊ���������ע�����⡣
��ú�� ����Ȼ������Ϊ��ʯȼ�ϡ�
��ȼú����ʱ����ú������ú�ۣ���Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ����ͭ��п�Ͻ�Ϊ�˲ⶨij��ͭ��Ʒ��п�������������ס��ҡ�����λͬѧ�ֱ����ʵ�飬ʵ���������£����ձ�������Ϊ56g��
| �� | �� | �� | |
| �ձ�+ϡ�������� | 139g | 129g | 129g |
| �����ͭ��Ʒ���� | 20g | 24g | 20g |
| ��ַ�Ӧ���ձ�+ʣ���������� | 158.8g | 152.8g | 148.8g |
��ش��������⣺
��1������ͬѧȡ�õ�ϡ�������Ʒǡ����ȫ��Ӧ������ȡ��������������������
��2�������ͭ��Ʒ��п������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�����˵����ȷ���� �� ��
 A�������ʵ��ܽ��һ�����������ʵ��ܽ��
A�������ʵ��ܽ��һ�����������ʵ��ܽ��
B��t20Cʱ���ס��ұ�����Һ�����������������
C��������Һ��t20C���µ�t10Cʱ��һ���о�������
D����t20Cʱ�ҵı�����Һ��Ϊ��������Һ���ɲ���
���µķ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��B��C��D���������ӵĽṹʾ��ͼ��E����Ԫ����Ԫ�����ڱ��е���Ϣ��

��ش��������⣺
��1��ͼ��A��B��C��D���ӹ���ʾ ��Ԫ�ء�
��2��A��B��C��D�б�ʾ�������������������γɵĻ������ ��ѧʽΪ ��
��ѧʽΪ ��
��3��D��x = ��
��4��E����Ԫ��ԭ�ӵ�������Ϊ ��һ����ԭ��������һ��̼12ԭ��������
 ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ�Ӧ�ò���ȷ���ǣ� ��

A��ѹ����Ȼ����ȼ�� B������ͭ�����Ƶ��� C����������ʳƷ���� D��ʯ��ʯ�к���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̫�ղ��Ϊ�˱��ֲ���O2��CO2���庬������ȶ�������NiFe2O4���������Ա������CO2ת��ΪO2��NiFe2O4����Ϊ+3�ۣ���Ni�Ļ��ϼ�Ϊ
A.+1 B. +2 C. +3 D .+4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ԫ���ڵؿ��к����ӵڶ�λ��������Ϣ���������Ͳ��ϵȷ�������Խ�Ĺ��ס�
��1����ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
��2��������������������ںͲ�ʿ�������S���ɹ��Ʊ����ྦྷ����̼���裨SiC���մɲ��ϣ���־�������������մ��о���������Ҫ��չ����֪SiC�й�Ԫ�صĻ��ϼ�Ϊ+4�ۣ���̼Ԫ�صĻ��ϼ�Ϊ ��
 ��3�����������������̼�Ļ�ѧ�������ƣ��������������������������Һ��Ӧ�Ļ�ѧ����ʽΪ
��3�����������������̼�Ļ�ѧ�������ƣ��������������������������Һ��Ӧ�Ļ�ѧ����ʽΪ  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
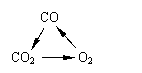
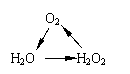
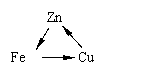 ���и���仯�У�ÿһת����һ�������¾���һ��ʵ�ֵ���
���и���仯�У�ÿһת����һ�������¾���һ��ʵ�ֵ���
�� �� ��
A���٢� B��ֻ�Т� C���ڢ�  D���٢ڢ�
D���٢ڢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com