



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
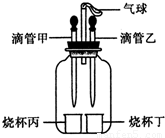
 ��2013?ƽ��ɽ��ģ����ͼ��ʾװ�ã����������ã����ı�ιܺ�С�ձ��е����ʿ�����ɶ����ʵ�飮
��2013?ƽ��ɽ��ģ����ͼ��ʾװ�ã����������ã����ı�ιܺ�С�ձ��е����ʿ�����ɶ����ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
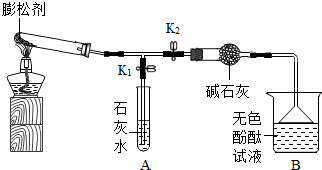 ��2013?�ϰ����ʼ죩���ɼ���һ��ʳƷ���Ӽ�������������������з�����Ӧ�������壬ʹ���������������ɼ���Ӧ�������������Ϊ�������ɼ�Ʒ�ʵ�һ����Ҫָ�꣮��֪ij���ɼ���̼�����ơ�̼����泥�NH4HCO3���е�һ�ֻ����֣���ѧ��ȤС�������������̽����
��2013?�ϰ����ʼ죩���ɼ���һ��ʳƷ���Ӽ�������������������з�����Ӧ�������壬ʹ���������������ɼ���Ӧ�������������Ϊ�������ɼ�Ʒ�ʵ�һ����Ҫָ�꣮��֪ij���ɼ���̼�����ơ�̼����泥�NH4HCO3���е�һ�ֻ����֣���ѧ��ȤС�������������̽����
| ||
| ||
| ʵ����� | ��Ҫʵ������ | ʵ����ۼ����� | |
| �� | ��ȼ�ƾ��ƣ� ��K1���ر�K2�� |
�Թ��а�ɫ������٣� ʯ��ˮ����� ʯ��ˮ����� |
�ж�����̼���ɣ�A�з�Ӧ�Ļ�ѧ����ʽΪ CO2+Ca��OH��2=CaCO3��+H2O CO2+Ca��OH��2=CaCO3��+H2O �� |
| �� | ��K2���ر�K1�� | ��ɫ��̪��Һ�� �� �� ɫ |
�а������ɣ� |
| �� | ������ּ��ȣ� | �Թ������й��� | �Թ����в��ֽ�Ĺ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
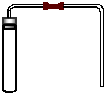 �÷��ӻ�ԭ�ӹ۵������������
�÷��ӻ�ԭ�ӹ۵�������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
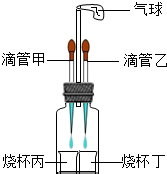
 ��2012?�人ģ�⣩��ͼ��ʾװ�ã����������ã����ı�ιܺ�С�ձ��е����ʿ�����ɶ����ʵ�飮
��2012?�人ģ�⣩��ͼ��ʾװ�ã����������ã����ı�ιܺ�С�ձ��е����ʿ�����ɶ����ʵ�飮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com