
| |||||||||||||||||||
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
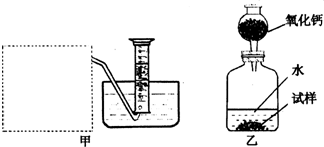
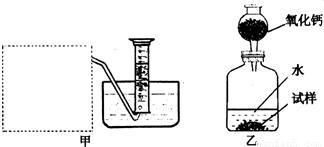
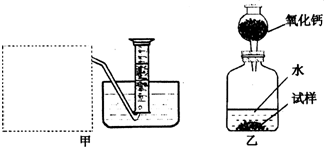 �Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
�Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ij� ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ƶ�������ͨ����������(Na2O)��������(Na2O2)��Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���塣Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
(1)�����Ѿ�֪����![]() �������Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ�� 2Na2O2+2H2O��4NaOH+ ���������������� ��
�������Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ�� 2Na2O2+2H2O��4NaOH+ ���������������� ��
(2)����ȤС���ͬѧ����˼ס�������װ�ã�

��װ�������߷�����Ϊ���巢��װ�ã�����ΪӦ��ʵ������ȡ ��װ����ͬ������ȤС��ѡ�����ַ����ռ������������ ������Ϊ�������� ���ռ���
(3)��װ�������ⶨ��������� ��
(4)��װ�������ⶨ��������� (��״����)����֪��������������Ҫ�����Na2O2�Ĵ��ȣ�����Ҫ֪���������� ��
A�������ڱ�״���µ��ܶ�
B����Ӧװ���м���ˮ������
C����Ӧ����Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006��ɽ��ʡ�ij����п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com