
ŅŃÖŖij“ÖŃĪѳʷ֊ŗ¬ÓŠÉŁĮæMgCl2”¢CaCl2¼°²»ČÜŠŌŌÓÖŹ”£Ä³ŃŠ¾æŠŌѧĻ°Š”×é¶ŌøĆ“ÖŃĪµÄĢį“æ½ųŠŠĮĖĢ½¾æ£¬Éč¼ĘČēĻĀŹµŃéĮ÷³Ģ£¬Ēėøł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ(20 ”ꏱ£¬²æ·ÖĪļÖŹµÄČܽāŠŌ¼ūĻĀ±ķ)
| Na£« | Mg2 | Ca2£« | |
| OH£ | ČÜ | ÄŃČÜ | Ī¢ČÜ |
| Cl£ | ČÜ | ČÜ | ČÜ |
| CO | ČÜ | Ī¢ČÜ | ÄŃČÜ |
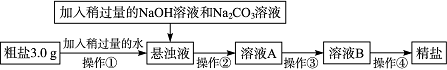
(1)²Ł×÷¢ŁÖŠŠčŅŖŹ¹ÓĆµÄ²£Į§ŅĒĘ÷ÓŠ£ŗÉÕ±”¢________”£²Ł×÷¢ŚµÄĆū³ĘĪŖ__________”£ČōŌŚ²Ł×÷¢Ś½įŹųŗó·¢ĻÖČÜŅŗAČŌ»ė×Ē£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ______________________”£
(2)²Ł×÷¢ŪŹĒŌŚ¼ÓČČĢõ¼žĻĀ²»¶ĻµĪ¼ÓĻ”ŃĪĖįÖĮČÜŅŗµÄpH£½7”£øĆ²Ł×÷µÄÄæµÄŹĒ________________________________________________________________________”£
(3)²Ł×÷¢ÜŹĒ½«ČÜŅŗBµ¹Čė______(ĢīŅĒĘ÷Ćū³Ę)ÖŠ£¬¼ÓČČ²¢²»¶Ļ½Į°č£¬Ö±µ½__________________________________(ĢīĻÖĻó)£¬Ķ£Ö¹¼ÓČČ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Na+ | Mg2+ | Ca2+ | |
| OH- | ČÜ | ÄŃČÜ | Ī¢ČÜ |
| Cl- | ČÜ | ČÜ | ČÜ |
| CO32- | ČÜ | Ī¢ČÜ | ÄŃČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
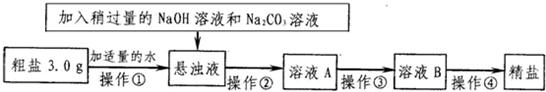
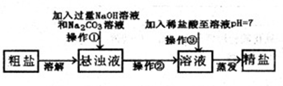 ŅŃÖŖij“ÖŃĪѳʷ֊ŗ¬ÓŠÉŁĮæMgCl2”¢CaCl2ŗĶ²»ČÜŠŌŌÓÖŹ£¬ĶعżČēĶ¼²½Ö襓ÖĘČ”¾«ŃĪ£®
ŅŃÖŖij“ÖŃĪѳʷ֊ŗ¬ÓŠÉŁĮæMgCl2”¢CaCl2ŗĶ²»ČÜŠŌŌÓÖŹ£¬ĶعżČēĶ¼²½Ö襓ÖĘČ”¾«ŃĪ£®| Na+ | Mg2+ | Ca2+ | |
| OH- | ČÜ | ÄŃČÜ | Ī¢ČÜ |
| Cl- | ČÜ | ČÜ | ČÜ |
| CO32- | ČÜ | Ī¢ČÜ | ÄŃČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com